双Salamo型四肟配体构筑的锌配合物:合成,晶体结构和荧光性质
杨玉华 郝静 董银娟 王刚 董文魁
(兰州交通大学化学与生物工程学院,兰州730070)
杨玉华 郝静 董银娟 王刚 董文魁*
(兰州交通大学化学与生物工程学院,兰州730070)
通过二水乙酸锌和双Salamo型四肟配体6,6′-二乙氧基-双(2,2′-(乙二氧双(氮次甲基)))四酚(H4L)的配位反应,合成了2种锌配合物即:[Zn3(L)(OAc)2(H2O)](1)和[Zn3(L)(OAc)2(H2O)]·[Zn3(L)(OAc)2(CH3OH)(H2O)]·3CH3OH·H2O(2)。该类配合物含有2个Salamo型L4-配体和3个锌离子,其中2个锌原子位于Salamo型螯合单元的N2O2空腔内。[Zn(L)]螯合物中桥联的酚氧原子进一步和中心的锌原子配位。这类结构能通过2个桥联的乙酸根配体稳定,从而使配合物1和2达到电荷平衡。配合物有2种不同的几何构型即扭曲的三角双锥和四方锥(配合物1)或三角双锥和八面体(配合物2)。另外,配合物1和2在激发波长为340和337 nm时能发出强的绿光,其最大发射波长分别为531和536 nm。
双Salamo型四肟配体;锌配合物;合成;晶体结构;荧光性质
0 Introduction
The salen-type ligand and its metal complexes were discovered since the 19th century,the synthesis of the salen-type complexes have been extensively investigated for the past several decades[1],At present, some significant applications in the research of the transitionmetal complexeswith salen-type ligands has been observed,and these complexes have been widely used in many areas,such as asymmetric catalysis[2], dioxygen carriers[3],Luminescent and magnetic properties[4-11]and biological activities,for instance,sterilization,anti-virusand anticancerand soon[12-20].Although the studies of salen-[21-25]or salamo-type[26-37]ligands and their complexes have made great progress,but it is very rarely that the study on bis(salen)-type ligands and theirmetal complexes[38-39].These bis(salen)-type ligands containing two salen-type moieties are also fascinating because some novel functions originating from the cooperation of several metal centers are expected.And these bis(salen)-type ligands play an important role in the selective strong binding with metal(ⅡorⅢ)atoms in coordination chemistry.These metal complexes also have some important practical values[40-41].
In order to further investigate syntheses,crystal structures and properties ofmetal complexes with bis (salen)-type ligands,herein,we report syntheses, structural characterizations and fluorescent properties of two new bis(salamo)-type Zncomplexes,[Zn3(L) (OAc)2(H2O)](1)and[Zn3(L)(OAc)2(H2O)]·[Zn3(L)(OAc)2(CH3OH)(H2O)]·3CH3OH·H2O(2)via the complexation of zincacetate dihydrate with a bis(salamo)-type tetraoxime ligand H4L.Furthermore,the fluorescence behavior of complexes 1 and 2 in DMF are discussed.
1 Experimental
1.1 M aterials and physicalmeasurements
3-Ethoxy-2-hydroxybenzaldehyde was purchased from Aldrich and used without further purification. Other reagents and solvents were analytical grade reagents from Tianjin Chemical Reagent Factory.C,H and N analyses were carried out with a GmbH VariuoEL V3.00 automatic elemental analyzer. Elemental analysis for Zn was detected by an IRIS ER/S·WP-1 ICP atomic emission spectrometer.1H NMR spectra were recorded on a Mercury-400BB spectrometer.UV-Vis absorption and fluorescence spectrawere recorded on a Shimadzu UV-2550 spectrometer and Perkin-Elmer LS-55 spectrometer,respectively.X-ray single crystal structures were determined on a Bruker Smart APEX CCD area detector.Electrolytic conductance measurements were made with a DDS-11D type conductivity bridge using a 1mmol·L-1solution in DMF at room temperature.Melting points were measured by the use of a microscopic melting point apparatus made in Beijing Taike Instrument Limited Company,and the thermometer was uncorrected.
1.2 Syntheses of H4L and its Zncomplexes 1 and 2
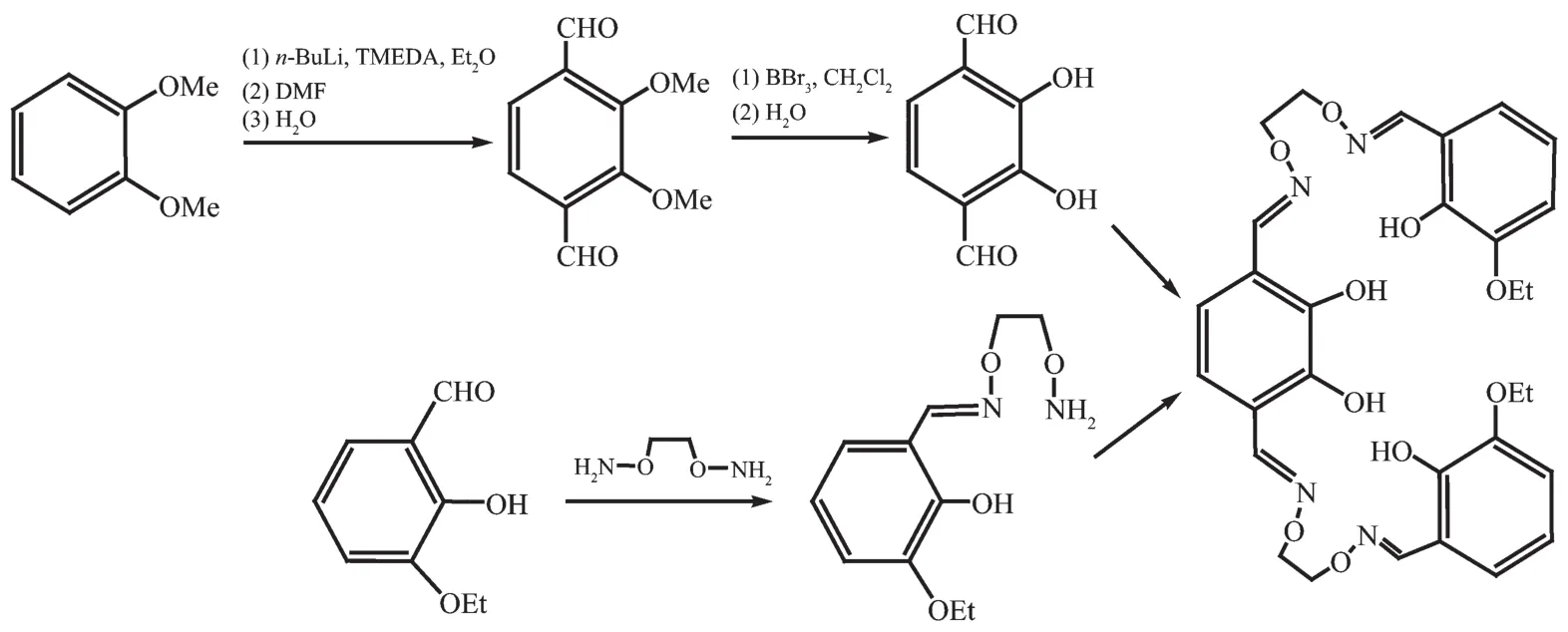
Scheme 1 Synthetic route to H4L
Synthetic route to H4Lis shown in Scheme 1.H4Lwas synthesized according to an analogous method reported earlier[39,42-45].m.p.401~402 K.1H NMR(400 MHz,CDCl3):δ=3.93(s,6H,-CH3),4.46~4.53(m,8H, -OCH2CH2O-),4.63(dt,J=5.4,1.5Hz,4H,-OCH2-),6.77 (s,2H,Ph-H),6.78~6.86(m,4H,Ph-H),6.93(dd,J= 7.2,2.2 Hz,2H,Ph-H),8.25(s,2H,N=CH),8.28(s, 2H,N=CH),9.60(s,2H,-OH),9.69(s,2H,-OH).Anal. Calcd.for C30H34N4O10(%):C,59.01;H,5.61;N,9.18. Found(%):C,59.15;H,5.57;N,8.96.
The single crystals of 2 were grown up by a similar procedure aforementioned taking Zn(OAc)2· H2O(3.26mg,0.015mmol)inmethanol(3mL)and H4L (3.03 mg,0.005 mmol)in 1.0 mL THF solution.The obtained bright yellow mixture was filtered and the filtrate was allowed to stand was stand at room temperature for three weeks.The solventwas partially evaporated and obtained yellow block-like single crystals suitable for X-ray crystallographic analysis. Yield:57.7%(3.4 mg).Anal.Calcd.for[Zn3(L)(OAc)2(H2O)]·[Zn3(L)(OAc)2(CH3OH)(H2O)]·3CH3OH·H2O (C72H93N8O35Zn6)(%):C,42.75;H,4.63;N,5.54;Zn, 19.40.Found(%):C,42.39;H,4.56;N,5.61;Zn,18.97. 1.3 Crystal structure determ ination
X-ray diffraction data were collected on a Bruker Smart Apex CCD diffractometer at 298(2)K. Using graphitemonochromatized Mo Kαradiation(λ= 0.071 073 nm).The structures were solved using the directmethod and refined by full-matrix least-squares on F2using the SHELXL-97 program package[46].All non-hydrogen atoms were refined anistropically and hydrogens were added in calculated positions and refined usinga ridingmodel.The X-ray crystallographic data collection,solution and refinement parameters for the Zncomplexes are summarized in Table 1.
CCDC:864890,1;1470489,2.

Table 1 X-ray crystallographic data and refinement parameters for comp lexes 1 and 2

Continued Table 1
2 Results and discussion
2.1 M olar conductance
Complexes 1 and 2 are soluble in DMF and DMSO,CHCl3,butnotsoluble in EtOH,MeOH,MeCN, THF,acetone and ethyl acetate.Complexes 1 and 2 display good stability in air at room temperature. Meanwhile,H4L is soluble in aforementioned solvents. Molar conductance values of complexes 1 and 2(1 mmol·L-1in DMF)at 298 K are 3.4 and 5.7 S·m2· mol-1,respectively,indicating complexes 1 and 2 are non-electrolyte.
2.2 FT-IR spectra
The IR spectra of H4L and its complexes 1 and 2 show a characteristic C=N stretching band.For the free ligand H4L this band appears at 1 608 cm-1, while the C=N bandsof complexes1 and 2 are observed at 1 616 and 1 612 cm-1,respectively.These shifts toward higher wavenumbers of the C=N absorption of about 8~6 cm-1on going from H4L to complexes 1 and 2 suggest a weak p-accepting ability of the coordinated ligand[47].
The Ar-O stretching frequencies appear as a strong band within the 1 265~1 213 cm-1range,as reported for similar salen-type ligands.These bands occur at 1 265 cm-1for H4L,1 260 cm-1for complex 1 and 1 263 cm-1for complex 2.The Ar-O stretching frequency is shifted to a lower value,indicating that the Zn-O bond was formed between the Znatoms and oxygen atoms of the phenolic groups[27-31,45].
The O-H stretching frequency of H4L appears at 3 410 cm-1.In addition,the broad absorption centered on 3 384,3 420 and 3 419 cm-1in complexes 1 and 2,respectively,which may be assigned to the O-H stretching vibration ofwater ormethanolmolecules.
2.3 UV-Vis absorption spectra
The absorption spectra of H4L and its complexes 1 and 2 in diluted DMF solution are shown in Fig.1. It can be seen that the absorption peaks of complexes 1 and 2 are obviously different from H4L upon complexation,and the spectral shapes of complexes 1 and 2 are similar to each other.An important feature of the absorption spectrum of H4L is shown that two absorption peaks are observed at 273 and 310 nm,respectively.The former absorption peak at 273 nm can be assigned to theπ-π*transition of the benzene rings and the latter one at 310 nm can be attributed to the intra-ligandπ-π*transition of the C=N bonds[28-29].

Fig.1 UV-Vis absorption spectra of H4L and its complexes 1 and 2 in DMF(50μmol·L-1)
Compared with the absorption peak of H4L,there are three absorption peaks in complexes 1 and 2.The former absorption peak at 273 nm in H4L was shifted to 285 and 287 nm in complexes 1 and 2,respectively. Meanwhile,the other absorption peak at 310 nm in H4L was shifted to 328 and 331 nm in complexes 1 and 2,which was shifted by ca.18 and 21 nm, respectively.The absorption peaks of 273 and 310 nm were red-shifted upon coordination to the Zn(II)atoms in complexes 1 and 2,which can be assigned to the π-π*transitions of the salamo-type ligand.In addition,a new absorption peak at 439 and 442 nmwas observed in complexes 1 and 2,respectively.
2.4 Description of the crystal structures
2.4.1 Crystal structure of complex 1
Selected bond lengths and angles for complex 1 are presented in Table 2.Complex 1 crystallizes in themonoclinic system,spacegroup P21/c.Theassembly of three Znatoms,one L4-ligand units,two acetate ions and one coordinated H2O molecule results in atrinuclear Zncomplex(Fig.2).In molecule unit of complex 1,three Znatoms are all pentacoordinated.Firstly,the terminal Znatom(Zn2)is penta-coordinated by two oxime nitrogen(N1 and N2) and phenolic oxygen(O1 and O5)atoms of the bis (salamo)-type L4-unit and one oxygen(O12)atom of one chelating acetate ion.Secondly,the centre Znatom(Zn1)is penta-coordinated by two bridging phenolic oxygen(O1 and O2)atoms of the bis(salamo) -type L4-unit,two oxygen(O11 and O13)atoms of the chelating acetate ions and one oxygen(O15)atom from one coordinated water molecule in the apical position.At last,The terminal Znatom(Zn3)is penta-coordinated by two oxime nitrogen(N3 and N4) and phenolic oxygen(O2 and O9)atoms of the bis (salamo)-type L4-unit and one oxygen(O14)atom ofthe chelating acetate ion.The two chelating acetate ions coordinate to the three Znatoms via a familiar Zn1-O-C-O-Zn2 and Zn1-O-C-O-Zn3 coordinatedmodes.
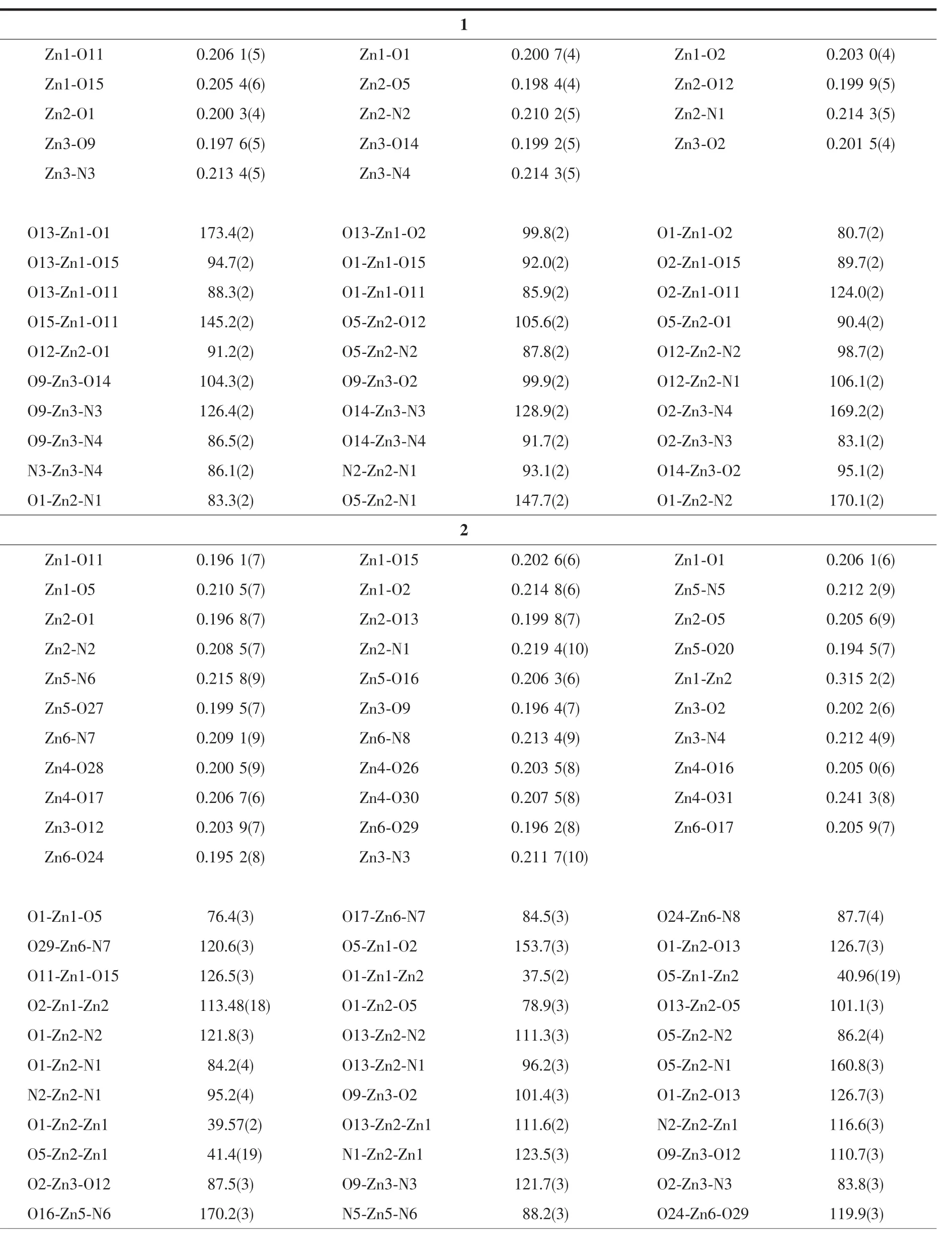
Table 2 Selected bond lengths(nm)and angles(°)for complexes 1 and 2
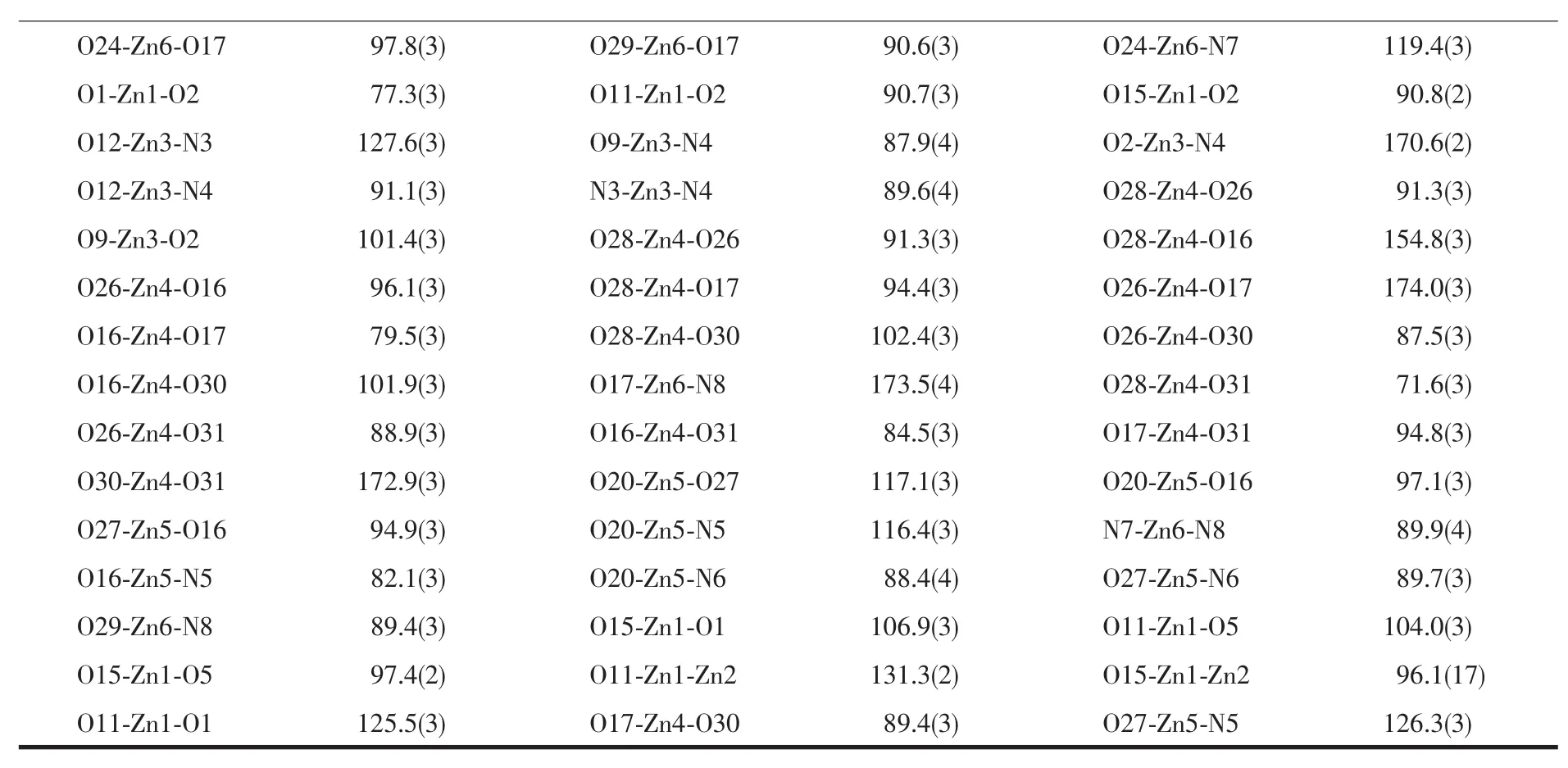
Continued Table 2
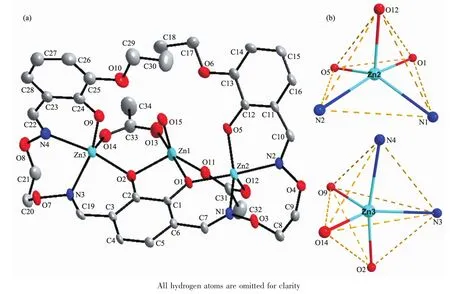
Fig.2 (a)Molecule structure and atom numberings of complex 1 with 30%probability displacementellipsoids; (b)Coordination polyhedra for Znatoms of complex 1
The Zn1 atom adopts a slightly distorted square pyramidal geometry(τ=0.470)[18],which deviate from themean plane(O1-O2-O11-O13)by 0.029 5(3)nm. The distance of Zn1 atom to the five donor atoms are all different(0.198 8(5)~0.206 1(5)nm).The distances from the four atoms to the mean plane are not equal (0.030 0~0.034 2 nm),and the distances from phenolic oxygen atom(O2)of the bis(salamo)-type L4-unit to themean plane is 0.221 8(4)nm.The Zn2 atom also adopts a slightly distorted square pyramidal geometry (τ=0.373),Four coordination atoms(O1,O5,N1 and N2)give a mean plane,and the Zn2 atom deviate from themean plane by 0.037 4(4)nm.The distances from the five coordination atoms to the mean plane are not equal:O1 and N2 above average by 0.021 3(3) and 0.019 1(3)nm,O5 and N1 below average by 0.020 8(4)and 0.019 6(3)nm,respectively.In addition, one oxygen atom(O6)of the acetate ion deviates from themean plane by 0.236 7(3)nm.The dihedral angle of O1-Zn2-N1 and O5-Zn2-N2 is 32.97(3)°.Interestingly,the geometry of the Zn3 atom is different from the Zn1 and Zn2 atoms.The value ofτ=0.671 clearly indicates that the environment of the Zn3 atom is a trigonal bipyramidal geometry in which the axial positions are occupied by O2 and N4 atoms.Which deviate from themean plane(O9-N3-O14)by 0.007 4(3) nm.The distance of the Zn3 atom to the five donor atomsarealldifferent(0.1976(5),0.1992(5),0.2015(4), 0.213 4(5)and 0.214 4(5)nm,respectively).The dihedral angle between the plane of N3-Zn1-O2 and thatof N4-Zn1-O9 is 53.10(3)°,which indicates the L4-unit has serious distortion.Thus,two kinds of type coordination geometries(trigonal bipyramidal and square pyramidal)are showed in complex 1.
2.4.2 Crystal structure of complex 2
Selected bond lengths and angles for complex 2 are presented in(Table 2).Complex 2 crystallizes in themonoclinic system,space group P21/c,Complex 2 consistsofsix Znatoms,two completely deprotonated L4-ligand units,four acetate ions,three coordinated watermolecules,three crystallizing methanol and one crystallizing water molecules.A perspective view of which is shown in Fig.3 together with the atomic labeling of the coordinated polyhedra.
As shown in Fig.3,the crystal structure of complex 2 consists of two independent molecules A and B,and the two molecules are all trinuclear structures.In molecule A,three Znatoms are all penta-coordinated.The Znatom(Zn1)is pentacoordinated by three phenolic oxygen(O1,O2 and O5) atomsof thebis(salamo)-type L4-unit,oneoxygen(O11) atom of the chelating acetate ion and one oxygen atom (O15)from one coordinated watermolecule.The Znatom(Zn2)is penta-coordinated by two oxime nitrogen (N1 and N2)and phenolic oxygen(O1 and O5)atoms of the bis(salamo)-type L4-unit and one oxygen(O13) atom of onemonochelate acetate ion.In addition,the Znatom(Zn3)is penta-coordinated by two oxime nitrogen(N3 and N4)and phenolic oxygen(O2 and O9)atoms of the bis(salamo)-type L4-unit and one oxygen(O12)atom of the chelating acetate ion.Itwill be seen from the discussion mentioned above that the coordination environments of the three Znatoms in themolecular A is very similar.
The coordination geometries of the Znatoms (Zn2 and Zn3)are best described as distorted trigonal bipyramidalgeometries(Zn2,τ=0.568;Zn3,τ=0.722)[18]in which the axial positions are occupied by O5,N1 and O2,N4 atoms,respectively,and the Zn2 and Zn3 atoms deviate from themean plane(O13-N2-O1)and (O12-N3-O9)by 0.006 0(3)and 0.001 2(3)nm,respectively.The distance of the Zn3 and Zn2 atoms to the five donoratomsarealldifferent(Zn2:0.196 8~0.219 4 nm;Zn3:0.196 4~0.212 4 nm).The dihedral angle between the plane of N2-Zn2-O5 and that of N1-Zn2-O1 is 58.74(3)°,and the dihedral angle between the plane of N3-Zn3-O2 and that of N4-Zn3-O9 is 58.44(3)°,which indicates the structureof the trinuclear core distorts from the ideal symmetry.Interestingly, the geometry of the centre Zn1 atom is different from the other two terminal Znatoms(Zn2 and Zn3). The Zn1 atom have a square pyramidal structure in which the axial sites are occupied by the O1 atom(τ=0.453),and which deviate from the mean plane(O1-O2-O11-O5)by 0.057 5(3)nm.The distance of the Zn1 atom to the four atoms from the mean plane are all different(0.196 1~0.214 8 nm).The distances from the four atoms to themean plane are not equal(0.026 1~0.041 4 nm),the dihedral angle between the plane of O1-Zn1-O5 and that of O2-Zn1-O11 is 53.83(3)°.In addition,there exists a four-membered ring(Zn1-O1-Zn2-O5)which adopts a chair-chair conformation. Thus,two kinds of coordination geometries(trigonal bipyramid and square pyramid)are showed in the molecule A.

Fig.3(a)Molecule structure and atom numberings of complex 2 with 30%probability displacementellipsoids; (b)Coordination polyhedra for Znatoms of complex 2
In themolecule B,Zn5 and Zn6 atoms are penta -coordinated,however,Zn4 atom is hexa-coordinated by two phenolic oxygen(O16 and O17)atoms of the bis(salamo)-type L4-unit,two oxygen(O26 and O28) atoms of the chelating acetate ions and two oxygen (O30 and O31)atoms from the coordinated water and methanol molecules,respectively.The coordination sphere of the terminal Znatom(Zn5)is completed by two oxime nitrogen(N5 and N6)and phenolic oxygen(O16 and O20)atoms of the bis(salamo)-type L4-unit and one oxygen(O27)atom of one chelating acetate ion.The coordination environment of the Zn6 atom is completely consistent with that of the Zn5 atom,and penta-coordinated by two oxime nitrogen (N7 and N8)and phenolic oxygen(O17 and O24) atoms of the bis(salamo)-type L4-unit and one oxygen atom(O29)of one chelating acetate ion.The two chelating acetate ions coordinate to the three Znatoms via a familiar Zn5-O-C-O-Zn4 and Zn4-O-C-OZn6 coordinatedmodes.
The Zn5 and Zn6 atoms have similar trigonal bipyramidalgeometries(Zn5,τ=0.732;Zn6,τ=0.882)[30]with approximatemolecular symmetry C3,in which the axial positions are occupied by O16,N6 and O17,N8 atoms,respectively.The Zn5 and Zn6 atoms deviate from the mean plane(O27-N5-O20)and(O24-N7-O29)by 0.004 8(3)and 0.003 0(3)nm,respectively. Although the Zn1 and Zn2 atoms are both penta-coordinated,but the distance of the Zn3 and Zn2 atoms to the five donor atoms are all different.The dihedral angle between the plane of N6-Zn5-O20 and that of N5-Zn5-O16 is 63.46(3)°,and another dihedral angle between the plane of N8-Zn6-O24 and that of N7-Zn6-O17 is 60.64(3)°.These results indicate that the L4-unithas serious distortion.Due to the Zn4 atom is hexa-coordinated,so it is clearly indicates that the coordination environment of the Zn4 atom is an octahedral geometry.The distance of the Zn4 atom to the six donor atoms are all different(Zn4-O28 0.200 5 nm,Zn4-O26 0.203 5 nm,Zn4-O16 0.205 0 nm,Zn4-O17 0.206 7 nm,Zn4-O30 0.207 5 nm and Zn4-O31 0.243 1 nm).In addition,The dihedral angle between the plane of O17-Zn6-O28 and that of O26-Zn4-O16 is 24.20(3)°.So,two kinds of coordination geometries (trigonal bipyramid and octahedron)are showed in the molecule B.
From the above,we can know complex 2 consists of two independentmolecules A and B,and the two molecules are all trinuclear structures.There are three kinds of coordination geometries(trigonal bipyramid, square pyramid and octahedron),and every trinuclear structure has serious distortion.
2.5 Supramolecular interaction
2.5.1 Supramolecular interaction of complex 1
The feature of complex 1 is its self-assembling array linked by intramolecular hydrogen bonds and intermolecular C-H…πinteractions.The hydrogen bond data and C-H…πinteraction data are given in (Table 3).
In the crystal structure,there are four intramolecular O15-H15C…O6,O15-H15C…O9,C9-H9B…O12 and C21-H21A…O14 hydrogen bonds (Table 3)involving the coordinated water,two acetate ions and alkoxy O atoms in each molecule,which is shown in Fig.4.There is also one intermolecular C-H…π(C9-H9A…Cg1)interaction(Table 3).The molecule is interlinked through intermolecular C-H…πinteractions into an infinite 1D chain(Fig.5).

Table 3 Intra-and intermolecular hydrogen geometries for com plexes 1 and 2
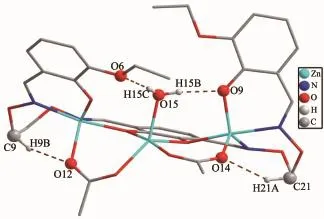
Fig.4 View of the intramolecular hydrogen-bonding interactions of complex 1
2.5.2 Supramolecular interaction of complex 2
In the crystal structure of complex 2,there are twelve intramolecular(O15-H15C…O9,O15-H15D…O14,O30-H30F…O24,O30-H30F…O24,O30-H30F…O25,O31-H31…O20,O33-H33…O35, O35-H35C…O34,C35-H35D…O25,C9-H9A…O13,C21-H21B…O12,C44-H44B…O27,C55-H55B…O29,C56-H56…O12,C71-H71A…O32 and C71-H71B…O26)hydrogen bond interactions(Table 3)involving three coordinated water,two acetate ions, crystallizing water molecules and alkoxy O atoms in each molecule,which is shown in Fig.6.Moreover, intramolecular C-H…π(C50-H50…Cg1)(Table 3) and hydrogen bonding interactions into an infinite wave-like 2D-layer supramolecular structure parallel to the crystallographic plane(Fig.7).

Fig.5 View of intermolecular C-H…πinteractions of complex 1
2.6 Lum inescence properties
Few reports have appeared so far on the prospective use of fluorescence characteristics on transition metal complexation of bis(salamo)-type tetraoxime ligands.In this work,the fluorescence studies have been employed as independent evidence of complexation between the ligand H4L and Znatoms.

Fig.6 View of the intramolecular hydrogen-bonding interactions of complex 2
The fluorescent properties of H4L and its complexes 1 and 2 were investigated at room temperature(Fig.8).The ligand H4L exhibits an intense emission peak at 456 nm upon excitation at 340 nm,which should be assigned to the intraligandπ-π*transition[19].The emission spectra of complexes 1 and 2 show amain peak at 531 nm(λex=340 nm) and 536 nm(λex=338 nm),respectively.The two Zncomplexes 1 and 2 exhibit similar fluorescence emissions because of their similar molecular structures. Meanwhile,it can be seen that complexes 1 and 2 exhibit a red-shiftwith respect to the bis(salamo)-type tetraoxime ligand H4L.We tentatively assign it to a ligand-to-metal charge transfer(LMCT)[31].In addition, compared with the emission spectrum of H4L,the enhanced fluorescence intensity of complexes 1 and 2 is observed,we attributed it to the following points: (1)themore rigidity of the ligand coordination to Znatom that effectively reduces the loss of energy and increase the emission efficiency;(2)full d10electronic configuration of Znatom;(3)An increased rigidity in structure of the complexes 1 and 2 and a restriction in the photoinduced electron transfer(PET)[48-49].In addition,The differences of the peak positionsmay be considered to be a result of the dissimilar coordination of themetal centers because the emission behavior is closely associated to the metal ions and ligands around them[31].The strong green fluorescence indicates complexes 1 and 2 may be a good candidate for fluorescentmaterials.Thus,the emission observed in complexes 1 and 2 is tentatively assigned to the LMCT fluorescence.
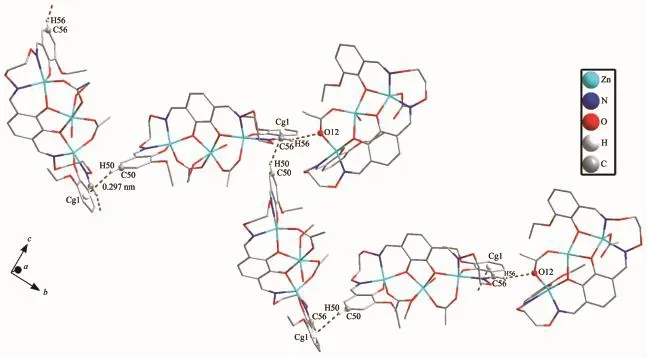
Fig.7 View of intramolecular and intermolecular C-H…πinteractions of complex 2

Fig.8 Emission spectra of H4L and its complexes 1 and 2 in dilute DMF solution(50μmol·L-1)at room temperature
3 Conclusions
Acknow ledgements:This work was supported by the National Natural Science Foundation of China(Grant No. 21361015),which is gratefully acknowledged.
[1]Costamagna J,Vargas J,Latorre R,etal.Coord.Chem.Rev., 1992,119:67-88
[2]Canali L,Sherrington D C.Chem.Soc.Rev.,1999,28:85-93
[3]Beck W M,Calabrese J C,Kottmair N D.Inorg.Chem., 1979,18:176-182
[4]Yu T Z,Zhang K,Zhao Y L,etal.Inorg.Chim.Acta,2008, 361:233-240
[5]Liu Y A,Wang C Y,Zhang M,et al.Polyhedron,2017,127: 278-286
[6]Liu PP,Wang C Y,Zhang M,et al.Polyhedron,2017,129: 133-140
[7]Song X Q,Liu P P,Liu Y A,et al.Dalton Trans.,2016,45: 8154-8163
[8]Song X Q,Zheng Q F,Wang L,et al.Luminescence,2012, 25:328-335
[9]Liu PP,Sheng L,Song X Q,et al.Inorg.Chim.Acta,2015, 434:252-257
[10]Song X Q,Liu PP,Xiao ZR,etal.Inorg.Chim.Acta,2015, 438:232-244
[11]Song X Q,Peng Y J,Chen G Q,et al.Inorg.Chim.Acta, 2015,427:13-21
[12]Han H Y,Song Y L,Hou H W,et al.J.Chem.Soc.,Dalton Trans.,2006,250:1972-1980
[13]Wu H L,Pan G L,Bai Y C,et al.Res.Chem.Intermed., 2015,41:3375-3388
[14]Wu H L,Wang C P,Wang F,et al.J.Chin.Chem.Soc., 2015,62:1028-1034
[15]Wu H L,Pan G L,Bai Y C,et al.J.Photochem.Photobiol. B,2014,135:33-43
[16]Wu H L,Bai Y C,Zhang Y H,etal.J.Coord.Chem.,2014, 67:3054-3066
[17]Chen C Y,Zhang JW,Zhang Y H,et al.J.Coord.Chem., 2015,68:1054-1071
[18]Wu H L,Bai Y C,Zhang Y H,et al.Z.Anorg.Allg.Chem., 2014,640:2062-2071
[19]Wu H L,Pan G L,Bai Y C,et al.J.Chem.Res.,2014,38: 211-217
[20]Wu H L,Pan G L,BaiY C,etal.J.Coord.Chem.,2013,66: 2634-2646
[21]Sun SS,Stern C L,Nguyen S T,et al.J.Am.Chem.Soc., 2004,126:6314-6326
[22]Zhao L,Dang X T,Chen Q,et al.Synth.React.Inorg.Met.-Org.Nano-Met.Chem.,2013,43:1241-1246
[23]XU Li(许力),ZHANG Yan-Ping(张艳萍),SHI Jun-Yan(史军妍),etal.Chinese J.Inorg.Chem.(无机化学学报),2007, 23:1999-2002
[24]Wang L,Ma JC,Dong W K,et al.Z.Anorg.Allg.Chem., 2016,642:834-839
[25]Xu L,Zhang Y P,Wang L,et al.Chin.J.Struct.Chem., 2008,27:183-186
[26]Wang P,Zhao L.Synth.React.Inorg.Met-Org.Nano-Met. Chem.,2016,46:1095-1101
[27]Sun Y X,Wang L,Dong X Y,et al.Synth.React.Inorg. Met-Org.Nano-Met.Chem.,2013,43:599-603
[28]DongW K,Ma JC,Zhu LC,etal.Inorg.Chim.Acta,2016, 445:140-148
[29]DongW K,Zhang J,Zhang Y,etal.Inorg.Chem.Acta,2016, 444:95-102
[30]DongW K,Li X L,Wang L,et al.Sens.Actuators B,2016, 229:370-378
[31]Ma J C,Dong X Y,Dong W K,et al.J.Coord.Chem., 2016,69:149-159
[32]Sun Y X,Zhang S T,Ren Z L,et al.Synth.React.Inorg. Met-Org.Nano-Met.Chem.,2013,43:995-1000
[33]Sun Y X,Gao X H,et al.Synth.React.Inorg.Met-Org. Nano-Met.Chem.,2011,4:973-978
[34]Dong X Y,Sun Y X,Wang L,et al.J.Chem.Res.,2012, 36:387-390
[35]Zhao L,Wang L,Sun Y X,et al.Synth.React.Inorg.Met-Org.Nano-Met.Chem.,2012,42:1303-1308
[36]Wang P,Zhao L.Spectrochim.Acta Part A,2015,135:342-350
[37]Xu L,Zhu L C,Ma JC,etal.Z.Anorg.Allg.Chem.,2015, 641:2520-2524
[38]Akine S,Taniguchi T,Nabeshima T,et al.Angew.Chem., 2002,114:4864-4867
[39]Chai L Q,Wang G,Sun Y X,et al.J.Coord.Chem.,2012,65:1621-1631
[40]Cho SH,Gadzikwa T,AfshariM,etal.Eur.J.Inorg.Chem., 2007:4863-4867
[41]Hoshino N.Coord.Chem.Rev.,1999,174:77-108
[42]DONGWen-Kui(董文魁),SHI Jun-Yan(史军妍),ZHONG Jin-Kui(钟金魁),etal.Chinese J.Inorg.Chem.(无机化学学报),2008,24:10-14
[43]DongW K,Sun Y X,He X N,et al.Spectrochim.Acta Part A,2010,76:476-483
[44]Akine S,Taniguchi T,Dong W K,et al.J.Org.Chem., 2005,70:1704-1711
[45]Dong W K,Sun Y X,Zhang Y P,et al.Inorg.Chim.Acta, 2009,362:117-124
[46]Sheldrick GM.Acta Crystallogr.Sect.A,2008,64:112-122
[47]Panja A,Shaikh N,Vojtišek P,et al.New J.Chem.,2002, 26:1025-1028
[48]Chattopadhyay N,Mallick A,Sengupta S.J.Photochem. Photobiol.A,2006,177:55-60
[49]Hennrich G,Sonnenschein H,Genger U R.J.Am.Chem. Soc.,1999,121:5073-5074
YANG Yu-Hua HAO Jing DONG Yin-Juan WANG Gang DONGWen-Kui*
(School of Chemical and Biological Engineering,Lanzhou Jiaotong University,Lanzhou 730070,China)
Two Zncomplexes,[Zn3(L)(OAc)2(H2O)](1)and[Zn3(L)(OAc)2(H2O)]·[Zn3(L)(OAc)2(CH3OH)(H2O)]· 3CH3OH·H2O(2)have been synthesized via the complexation of zincacetate dihydrate with a bis(salamo)-type tetraoxime ligand H4L(H4L=6,6′-diethoxy-bis(2,2′-(ethylenedioxybis(nitrilomethylidyne)))tetraphenol).X-ray crystallographic analyses reveal formation of trinuclear structures consisting of two salamo-type L4-ligands and three Znatoms as expected from the analytical data.Two of the three Znatoms are located in the salamotype L4-chelatemoieties.Theμ-phenoxo oxygen atoms of the[Zn(L)]chelates further coordinate to centre Znatom.The trinuclear structure is probably stabilized by the twoμ-acetato ligands,which neutralize the whole charge of the complexes 1 and 2.There are two kinds of coordination geometries(trigonal bipyramidal and square pyramidal or trigonal bipyramidal and octahedral geometries)in complexes 1 and 2.In addition,complexes 1 and 2 exhibit strong green emissionλmax=531 and 536 nm when excited with 340 and 337 nm,respectively.CCDC: 864890,1;1470489,2.
bis(salamo)-type tetraoxime ligand;Zncomplex;synthesis;crystal structure;luminescence property
O614.24+1
A
1001-4861(2017)07-1280-13
10.11862/CJIC.2017.150
2017-03-17。收修改稿日期:2017-05-08。
国家自然科学基金(No.21361015)资助项目。
*通信联系人。E-mail:dongwk@126.com;会员登记号:02M87091161。

