Co3O4/ZnO修饰针灸针的制备及其在葡萄糖检测领域的应用
苑鸿雯 王玉路 马驰 耿俊隆 张利强 崔海*,
(1首都医科大学中医药学院,中医络病研究北京市重点实验室,北京100069)(2内蒙古科技大学,包头医学院第一附属医院,包头014010)(3中国石油大学(北京)重质油国家重点实验室,北京102249)
Co3O4/ZnO修饰针灸针的制备及其在葡萄糖检测领域的应用
苑鸿雯1王玉路2马驰3耿俊隆1张利强3崔海*,1
(1首都医科大学中医药学院,中医络病研究北京市重点实验室,北京100069)
(2内蒙古科技大学,包头医学院第一附属医院,包头014010)
(3中国石油大学(北京)重质油国家重点实验室,北京102249)
将Co3O4/ZnO针状纳米棒材料修饰到针灸针表面用于检测葡萄糖浓度的变化。首先采用水热法在针灸针表面得到Co(CO3)0.5(OH)·0.11H2O针状纳米棒前驱体,然后在500℃条件下退火3 h得到Co3O4针状纳米棒阵列。再采用浸渍法将预制备好的ZnO量子点修饰到Co3O4针状纳米棒表面,得到Co3O4/ZnO复合修饰的针灸针。研究发现此针灸针对葡萄糖具有较好的电流响应(2 264.27μA·L·mmol-1·cm-2)、较快的响应速度(<4 s)及较低的检测极限(0.311μmol·L-1(S/N=3))。且该针灸针在用于检测人体模拟细胞液中葡萄糖浓度时,对抗坏血酸和尿素等表现出较强的抗干扰性。
Co3O4;ZnO;针灸针;葡萄糖;传感器
0 Introduction
Acupuncture is good at prophylaxis and treating some certain chronic diseases[1-2].In the process of acupuncture,acupoints are the main sites of action, which are closely related to the efficacy[3-4].However, there is no or even negative effect if we needling on the non-acupoints.Exploring the specific differences between the acupoints and non-acupoints is always a hot spot in modern Chinese medicine research[5-6]. According to the metabolomics statistics,it is found that the efficacy of acupuncture has a close relationship with the metabolites changes upon external stimuli,but their specific relationship is not clear,greatly confining evaluating the efficacy of acupuncture[7].Therefore,quantitatively comparing the biological signals changes on acupoints and nonacupoints upon acupuncture is significant in the study of acupuncture.
A variety of methods have been tried to study biological changes produced upon acupuncture, including positron emission tomography(PET), functional magnetic resonance imaging(fMRI)brain imaging,blood biochemical indices analyze,etc[8-9].It has been found there exists a significant different brain imaging between acupoints and non-acupoints, and the images from acupoints have a certain physiological specificity[10].Although the existence of differences between acupoints and non-acupoint has already been confirmed,it stays only in a qualitative level and the specific diversity is still unknown[11].Moreover,the physiological indictor difference between acupoints and non-acupoints may last only for a short time, which is hard to detect and record.Till now,making an in situ,real-time,quantitative detection of the physiological signal changes upon acupuncture is still quite difficult.
In recent years,it is found nano biosensors can detect human metabolism product changes with a fast speed,high efficiency and good accuracy[12-13].Supposing adding these nano-biosensors during the acupuncture treatment,it may provide a new way for quantitative characterizing biological signals changes on acupoints and non-acupoints upon acupuncture.In all types of biological indicators,charactering the glucose concentration changes is considered as the easiest and most widely used indicator for a clinical practice[13-14].Various types of glucose biosensor were designed,and wherein the nano-cobalt oxide(Co3O4) based biosensors developed rapidly due to its fast response,good anti-interference,wide detection range, and high stability[15-16].Although various types of nano-Co3O4(nano-films,nanorods,nanotube,etc.)based biosensorhave been prepared[17-20],no reportshave tried fabricating Co3O4acicular nanorod arrays(ANRAs)on the surface of acupuncture needles for making an in situ,real-time,quantitative charactering glucose concentration changes.Furthermore,in order to improve the performance of Co3O4,ZnO quantum dots (QDs)could be further decorated on the surface of Co3O4ANRAs to form a p-n junction.
Therefore,in this study Co3O4/ZnO ANRAs were fabricated on the surface of acupuncture needles by using hydrothermal method.And then,glucose concentration changes were quantitatively monitored through electrochemistry technique by using this acupuncture needle.The Co3O4/ZnO decorated acupuncture needle demonstrates a good glucose sensing ability,with a fast response,good antiinterference,wide detection range,and high stability.
1 Experimental
In a typical procedure of fabricating the Co3O4ANRAs,4 mmol(1.16 g)of cobalt nitrate(Co(NO3)2· 6H2O),8 mmol(0.29 g)ammonium fluoride(NH4F) and 8 mmol(1.12 g)of urea(CO(NH2)2)as sources were dissolved in 40 mL deionized water under stirring at room temperature.After stirring for 20 min, the homogeneous solution was transferred into a 100 mL Teflon lined stainless steel autoclave.An acupuncture needle was immersed in the reaction solution against the bottom of the autoclave.In order to collect enough Co3O4powders for N2adsorptiondesorption,a piece of cleaned stainless steel foil(80 mm×20mm)was also immersed in the autoclave.The autoclave was sealed and maintained in an electricoven at95℃for 24 h.After cooling down to the room temperature,the acupuncture needle was took out and rinsed with distilled water for several times,and dried at 60℃in vacuum for 2 h.The as-prepared precursors of Co(CO3)0.5(OH)·0.11H2O were converted to Co3O4via thermal decomposition at 500℃in the air for 3 h.
The ZnO QDs were fabricated by dissolving 1.10 g of Zn(Ac)2·2H2O in 50mL of boiling ethanol.After fully dissolving,the solution was cooled down to 0℃, and a white powder was precipitated on the bottom. At the same time,LiOH·H2O(0.29 g)was dissolved in 50mL ethanol at room temperature in an ultrasonic bath and the system was cooled to 0℃.Then the LiOH solution was added to the Zn(Ac)2suspension dropwise under vigorous stirring at 0℃until the reaction mixture became transparent.The obtained ZnO QDs solution needs to be stored below 4℃to prevent rapid particle growth.To washing the ZnO solution,ZnO sol and hexane were mixed in the volume ratio of 1∶1.The supernatant was removed by decantation or centrifugation.The ZnO precipitate was re-dispersed in ethanol.This procedure could be repeated several times.After that,ZnO QDs were coated on the Co3O4via a facile soakmethod.
The structure of sample was characterized by using Bede D1 X-ray diffraction(XRD)system with a Cu Kαradiation in the 2θrange of 20°~80°(λ= 0.154 06 nm,U=40 kV,I=40mA).Surfacemorphology of all the samples and the element contains were studied by using the FEI Quanta 200F scanning electron microscope(SEM)operated at a voltage of 20 kV equipped with energy-dispersive X-ray spectroscopy (EDX).The X-ray photoelectron spectroscopy(XPS, Thermo Fisher K-Alpha American with an Al KαX-ray source)was used to characterize the Co3O4ANRAs as well.Specific surface areas of the sample was obtained from the results of N2adsorption-desorption isotherms at 77 K(Micromeritics ASAP 3020)after using Brunauer-Emmet-Teller(BET)method.The structure of the samples is identified by using the FEI Tecnai G2 F20 transmission electron microscopy (TEM),which is operated at 200 kV.Themicroscopic Raman spectroscopy was recorded by using a micro-Raman spectrometer(InVia Reflex,Renishaw,UK) with excitation wavelength of 532 nm.All peaks in the Raman spectra were fitted with Lorentzians.All electrochemical measurements,including cyclic voltammetric(CV)and amperometric experiments(I-t) were performed with a CHI 760E electrochemical workstation using the conventional three-electrode system.The Co3O4/ZnO decorated acupuncture needle, a platinum sheet,and an Ag/AgCl/KCl electrode were used as the working electrode,counter electrode,and reference electrode,respectively.All electrochemical measurements were conducted in the 1.0 mol·L-1NaOH aqueous solution at room temperature.
2 Results and discussion
As we known,an acupuncture needle can only be used for treating symptoms in the past.However, herein we prepare homogeneous and stable layers of Co3O4/ZnO ANRAs on the tip surface of acupuncture needle,adding another significant biosensing function on the traditional acupuncture needle.It is expected that Co3O4/ZnO ANRAsmodified acupuncture needle can be applied to characterize the biological signals changes through electrochemistry technique,as illustrated in Fig.1.
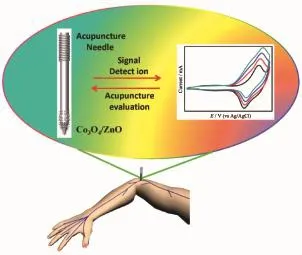
Fig.1 Conceptive illustration of applying Co3O4/ZnO modified acupuncture needle for quantitative detecting of the biological index changes upon acupuncture through electrochemistry technique
The fabrication process of Co3O4/ZnO ANRAs on the surface of acupuncture needle was schematicallyillustrated in Fig.S1.Firstly,Co related precursor arrays were obtained on acupuncture needle through hydrothermal method by using the Co(NO3)2·4H2O, NH4F,and CO(NH2)2as sources at 95℃for 24 h.In order to improve the binding force between the Co3O4and acupuncture needles,the surface roughness of acupuncture needle was enhanced prior to the experiment,including sanding with fine sandpaper and then coating with nano gold.After hydrothermal reaction,the surface of the substrate would be covered with pink precursors,which can be fully converted to the black Co3O4via calcination at 500℃for 3 h.And then,ZnO quantum dots(QDs)were decorated on the surface of Co3O4ANRAs through dipping technique. Finally,an acupuncture needle equipped with biosensing function could be obtained by sealing the end of the needlewith a little epoxy resin.
The XRD pattern of as-synthesized Co3O4/ZnO ANRAs deposited on the surface of acupuncture needle is shown in Fig.2.As the amount of Co3O4/ZnO NW on a single needle is not enough for the XRD characterization,we collect the Co3O4/ZnO powder from twenty needles.In the XRD pattern of the prepared Co3O4,all the diffraction peaks can be indexed to the face-centered cubic Co3O4(PDF#43-1003),which confirms the complete conversion from cobalt carbonate hydroxide to Co3O4.As the amount of ZnO is quite small,we did not find any peaks related to ZnO from the XRD pattern.No other impurity was found from the XPS spectrum of the as-prepared Co3O4(Fig.S2).The four obvious vibration bands located at 475,518,615 and 686 cm-1in the Raman spectrum also confirmed the good crystal quality of Co3O4ANRAs(Fig.S3)[19].
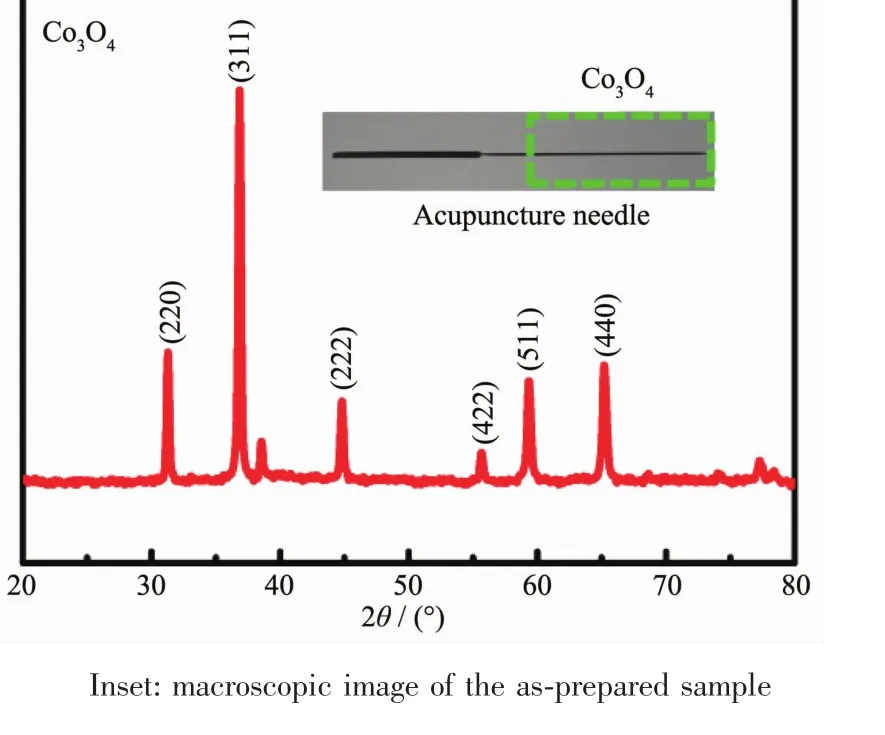
Fig.2 XRD pattern of the Co3O4/ZnO fabricated on the surface of acupuncture needle

Fig.3(a)SEM image of the Co3O4/ZnO ata low magnification;(b)Highmagnification SEM image of the pristine Co3O4, in which the Co3O4ANRAs are homogeneously aligned on the needle like a cactus ball;(c)TEM image of ZnO QDs ata highmagnification;(d)Typical TEM image of Co3O4/ZnO in a highmagnification;(e)Diffraction pattern of Co3O4/ZnO ANRAs;(f)STEM image and related EDX mapping of a single Co3O4/ZnO ANRA
The morphology of the Co3O4/ZnO ANRAs was characterized by SEM and TEM(Fig.3).In Fig.3(a),alarge scale and high density Co3O4arrays are found uniform ly deposited on the acupuncture needle.From the high magnification SEM image of Co3O4(Fig.3(b)), we found that the Co3O4arrays demonstrate an acicularmorphology.Fig.3(c)demonstrates a typical TEM image of the prepared ZnO QDs.The high magnification of Co3O4/ZnO is shown in Fig.3(d),from which an obvious acicular like Co3O4ANRAs was observed,and the length of Co3O4ANRAs were~1 μm.After annealing,plenty of nanopores were formed in the Co3O4ANRAs,which may be generated due to the dehydration and lattice contraction occurring during the thermal treatment.These nanopores existed in the Co3O4lead to a relative large SBET(~20 m2·g-1) compared to that of the as-prepared precursor(~3m2· g-1).From Fig.3(d),it is also found that Co3O4ANRAs are not a single crystal but composed by a lot of nanocrystals,which own a large surface accompanying with plenty of highly active surfaces exposed.Fig.3(e) shows the diffraction pattern of the Co3O4/ZnO ANRAs, in which the circleswere attributed to Co3O4/ZnO.Fig. 3(f)shows the STEM image of the Co3O4/ZnO,from which it is found that the ZnO QDs are uniformly decorated on the surface of Co3O4.
The electrochemical behaviors of the modified electrode were studied by cyclic voltammetry(CV). Fig.4(a)compares the CV curves of the bare needle, Co3O4decorated needle,and Co3O4/ZnO ANRAs modified acupuncture needle in the potential range of -0.1~0.6 V(vs Ag/AgCl)at a scan rate of 40 mV·s-1. The experiments were carried out in alkaline solution (1.0mol·L-1NaOH).It is found that there are not any peaks for the bare needle.While for the Co3O4,two pairs of redox peaks appear,which could be further improved after decorated with ZnO.Herein,a typical p-n heterojunction is formed when n-type ZnO is decorated on p-type Co3O4,which improves its biosensing response.The electrochemical performance of pure ZnO electrode for detecting glucose is shown in Fig.S4,in which almost no current response can be observed after the addition of glucose.Since ZnO has almost no response to the glucose,we only explore the behavior of Co3O4.The first pair of redox peaks appeared at around 0.1 V(Fig.4(a),Inset)can be ascribed to the reversible transition between Co3O4and CoOOH(Reaction 1),and the other redox peak (0.5 V)can be assigned to the conversion of CoOOH and CoO2(Reaction 2)[21].These two reversible reactions can be represented by the following equations:

Fig.4(a)CV curves of the bare acupuncture needle, Co3O4and Co3O4/ZnO ANRAsmodified acupuncture needle electrode in the presence and absence of glucose;(b)Co3O4/ZnO electrode at different scan rates;(c)CV curves of the Co3O4/ZnO ANRAsmodified acupuncture needle electrode in the presence of glucose with different concentrations
Upon the injection of glucose(concentration of glucose in alkaline solution is 1 mmol·L-1),the CV curves of Co3O4quickly shift to more positive values especially in the anodic peaks.With the increased scan rate from 10 to 100 mV·s-1,the current density demonstrates an apparent higher response,indicating a surface-controlled process of redox reaction of Co3O4on acupuncture needle(Fig.4(b)).The liner response behavior(Fig.4(b),Inset)manifests that the modified acupuncture needle might be used for quantitative detecting the concentration of glucose in situ.The biosensing mechanism can be roughly summed up through the following equation 3 of the electrochemical oxidation reaction from glucose to gluconolactone catalyzed by Co3O4[22]:
CoO2+glucose→CoOOH+gluconolactone(3)
As this reaction proceeds,CoO2attendant are consumed,contributed to the occurrence of the positive reaction of equation 2,which synchronously leads the current density increases and results in the detection of the addition of glucose(Fig.4(c)).

Fig.5(a)Amperometric response to successive addition of glucose obtained by using Co3O4/ZnO electrode at an applied potential of 0.55 V;(b)Corresponding calibration curves from(a);(c)Amperometric response of Co3O4/ZnO electrode to successive addition of 0.2mmol·L-1glucose with intervals of 100 s,and 0.02mmol·L-1AA,serum and 0.04mmol· L-1UA were respectively injected to the NaOH solution(1.0mol·L-1)in themiddle of each interval;(d)Current density response columnar diagram of the tested analytes compared with glucose
Fig.5(a)displays the amperometric responses of Co3O4and Co3O4/ZnO ANRAs electrode to successive addition of glucose with serial concentrations.The suitable sensing potential is determined to be 0.55 V from the CV curves,and a current density-time plot was obtained by the successive addition of different concentration of glucose solution into 1.0 mol·L-1NaOH solution.The initial rate for the addition of glucose is 0.2 mmol·L-1till the total glucose concentration in alkaline solution reached 1 mmol·L-1, during which the glucose increment wasmade at the rate of 0.2 mmol·L-1,and each rate dropped five timeswith an interval of 50 s till the end.The starting points of additions of glucose at different rate are noted by black arrows in Fig.5(a).It is found that Co3O4/ZnO ANRAs demonstrate a higher response tothe addition of glucose than Co3O4.To further study the amperometric performance of Co3O4/ZnO ANRAs electrode,a calibration curve for glucose is constructed by evaluating the fluctuation in current density with each addition of glucose solution at a certain concentration shown in Fig.5(b).The linear range of Co3O4/ ZnO electrode′s glucose sensing is 0.2~3.0 mmol·L-1(R=0.997 7),and the linear relationship between the response current and the glucose concentration is given by the equation y=2 264.27x+602.53(x is the glucose concentration(mmol·L-1),y is the response current(μA)).Besides,from the slope of the two fitted curves,the sensitivity of Co3O4/ZnO electrode was determined to be 2 264.27μA·L·mmol-1·cm-2.A rapid response time(<4 s)and a remarkable low detection limit of 0.311μmol·L-1(S/N=3)are also interpreted from Fig.5(b),which are also better than those of the pristine Co3O4.
The selectivity of the Co3O4/ZnO ANRAs was investigated against ascorbic acid(AA),serum and uric acid(UA).The amperometric detection was conducted at the potential of 0.55 V in 1 mol·L-1NaOH solution under stirring.After the current density of the curve leveled off,0.2mmol·L-1(glucose concentration in total NaOH solution)glucose was continuous added to the NaOH solution at intervals of 100 seconds.In the middle of the interval,0.02 mmol·L-1AA,pure serum,0.04 mmol·L-1UA were successive injected to the NaOH solution.As shown in Fig.5(c),stepped curve remained intact form after the addition of interferent,which indicates that AA, pure serum and UA will not prevent the selective detection of glucose.What is noteworthy is that there are a large number of different categories of proteins and biomolecules in the serum,the Co3O4electrode can still keep its highly resistant to interference.The current density response columnar diagram of each kind of interferent compared with glucose is demonstrated clearly in Fig.5(d),which confirms the highly anti-interference ability of Co3O4/ZnO ANRAs electrode.A comparison of the Co3O4/ZnO ANRAs composite with some of the reported non-enzymatic glucose biosensors based on metal oxides or metal hydroxides is summarized in Table 1[16-17,20,23-28].It is found that the Co3O4/ZnO ANRAs exhibit satisfactory performances including high sensitivity,low detection limit and wide linear range.In particular,it has obviously higher sensitivity and lower detection limit than those reported previously.
Additionally,we tried to use this acupuncture needle to characterize the glucose with unknown concentration in an assumed solution.Through measuring the current(2.4 mA),it is calculated the concentration of glucose is 1.07mmol·L-1,which is in a relatively agreement with the presetting concentration(1 mmol·L-1).Additionally,the concentration of glucose was in situ changed by adding more glucose into the solution or dissolving it with more water.In this process,the current occurred a rapid response, proving that this acupuncture needle might make anin situ,real-time,quantitative charactering the glucose concentration changes.

Table 1 Com parison of as-prepared sensorsw ith other reported glucose biosensors in performance
3 Conclusions
In summary,a novel acupuncture needle with biosensing function was fabricated by coating with Co3O4/ZnO ANRAs on its surface through a hydrothermal method.The structure and morphology of Co3O4/ZnO were characterized by XRD,SEM,and TEM,which confirmed that Co3O4/ZnO ANRAs were uniformly distributed on the surface of acupuncture needle.This bio-functional acupuncture needle demonstrates excellent sensitivity,fast amperometric response,low detection limit and good selectivity for the detecting of glucose.The large specific surface area provided by Co3O4/ZnO ANRAs and the good sensing abilities of Co3O4contribute to its excellent performance.The success of adding the biosensing function on the acupuncture needle provide a novel way to in situ monitoring biological indicator concentration changes upon acupuncture,which is significant for quantitative evaluating and understanding the clinical efficacy of acupuncture in modern Chinese medicine.
Acknow ledgments:YUAN Hong-Wen and WANG Yu-Lu contribute equally to this work.This work is financially supported by the National Natural Science Foundation of China (Grants No.81403467,51401239).
Supporting information isavailable athttp://www.wjhxxb.cn
[1]Raith W,Li Y,Zheng H,et al.Dtsch.Z.Akupunktur,2012, 55(4):22-24
[2]Napadow V,Ahn A,Longhurst J,etal.J.Altern.Complement Med.,2008,14(7):861-869
[3]Kaptchuk T J.Ann.Int.Med.,2002,136(5):374-383
[4]Leake R,Broderick JE.Integr.Med.,1999,1(3):107-115
[5]Liu C Z,Xie JP,Wang L P,et al.Pain Med.,2014,15(6): 910-920
[6]Ma T T,Yu S Y,Li Y,et al.Aliment.Pharmacol.Ther., 2012,35(5):552-561
[7]Fuentealba Cargill F,Biagini Alarcón L.Rev.Med.Chile, 2016,144(3):325-332
[8]Zhao ZQ.Prog.Neurobiol.,2008,85(4):355-375
[9]Mayer D J.Progress in Brain Research.Vol.122.Mayer E A, Saper C B Ed.,Amsterdam:Elsevier,2000:457-477
[10]Li L,Liu H,Li Y Z,et al.J.Altern.Complement Med., 2008,14(6):673-678
[11]Garcia M K,McQuade J,Haddad R,et al.J.Clin.Oncol., 2013,31(7):952-960
[12]Anker JN,HallW P,Lyandres O,et al.Nat.Mater.,2008, 7(6):442-453
[13]Tian K,Prestgard M,Tiwari A.Mater.Sci.Eng.C,2014,41: 100-118
[14]Scognamiglio V.Biosens.Bioelectron.,2013,47:12-25
[15]Ding Y,Wang Y,Su L,etal.Biosens.Bioelectron.,2010,26 (2):542-548
[16]Khun K,Ibupoto ZH,Liu X,etal.Mater.Sci.Eng.B,2015, 194:94-100
[17]Gao Z,Zhang L,Ma C,et al.Biosens.Bioelectron.,2016, 80:511-518
[18]Fan S,Zhao M,Ding L,et al.J.Electroanal.Chem.,2016, 775:52-57
[19]Zhang L,Gao Z,Liu C,et al.J.Mater.Chem.A,2015,3(6): 2794-801
[20]Li M,Han C,Zhang Y,et al.Anal.Chim.Acta,2015,861: 25-35
[21]Casella IG,Gatta M.J.Electroanal.Chem.,2002,534(1):31 -38
[22]Heli H,Yadegari H.Electrochim.Acta,2010,55(6):2139-2148
[23]Wang X,Dong X,Wen Y,et al.Chem.Commun.,2012,48 (52):6490-6492
[24]Yin H,He X,Cui Z,et al.IET Micro Nano Lett.,2016,11 (3):151-155
[25]LiSJ,Hou L L,Yuan BQ,etal.Microchim.Acta,2016,183 (6):1813-1821
[26]Bao L,Li T,Chen S,et al.Small,2017,13(5):DOI:10.1002/ sm ll.201602077
[27]Chen T,Li X,Qiu C,et al.Biosens.Bioelectron.,2014,53: 200-206
[28]Wu M Y,Meng S J,Wang Q,et al.ACS Appl.Mater. Interfaces,2015,7(38):21089-21094
Co3O4/ZnO M odified Acupuncture Needle:Preparation and Application in Detecting Glucose
YUAN Hong-Wen1WANG Yu-Lu2MA Chi3GENG Jun-Long1ZHANG Li-Qiang3CUIHai*,1
(1School of Chinese Medicine,Beijing Key Lab of TCM Collateral Disease Theory Research, CapitalMedical University,Beijing 100069,China)
(2The First Affiliated Hospital of Baotou Medical College of Inner Mongolia University of Science and Technology,Baotou,Inner Mongolia 014010,China)
(3State Key Laboratory of Heavy Oil Processing,China University of Petroleum-Beijing,Beijing 102249,China)
A Co3O4/ZnO acicular nanorod arrays(ANRAs)decorated acupuncture needle has been fabricated,which demonstrates a good performance for detecting the glucose.Firstly,the precursor Co(CO3)0.5(OH)·0.11H2O ANRAs were fabricated on the acupuncture needle through hydrothermal method.Thereafter,they could be completely converted to the Co3O4ANRAs through annealing at500℃for 3 h in the air,then ZnO quantum dots(QDs)were decorated on the surface of Co3O4ANRAs through dipping technique.It is found that Co3O4/ZnO ANRAs decorated acupuncture needle exhibits a good current response to glucose(2 264.27μA·L·mmol-1·cm-2), meanwhile owning a fast response time(<4 s)and a detection limit as low as 0.311μmol·L-1(S/N=3).Moreover, this acupuncture needle exhibited a high selectivity for glucose in human serum,against ascorbic acid and uric acid.
Co3O4;ZnO;acupuncture needle;glucose;sensor
O614.81+2;O614.24+1;R245-33
A
1001-4861(2017)07-1139-08
10.11862/CJIC.2017.118
2016-12-03。收修改稿日期:2017-04-19。
国家自然科学基金(No.81403467,51401239)资助项目。*
。E-mail:drcuihai@163.com

