含C^N-螯合配体的环戊二烯基铱抗癌配合物
路小敏 田梦 田珍珍 田来进 李梦琪 黄静 刘哲
(曲阜师范大学化学与化工学院,抗癌药物开发与诊疗应用研究所,山东省生命有机分析重点实验室,山东省绿色天然产物及医药中间体开发重点实验室,曲阜273165)
含C^N-螯合配体的环戊二烯基铱抗癌配合物
路小敏 田梦 田珍珍 田来进 李梦琪 黄静 刘哲*
(曲阜师范大学化学与化工学院,抗癌药物开发与诊疗应用研究所,山东省生命有机分析重点实验室,山东省绿色天然产物及医药中间体开发重点实验室,曲阜273165)
合成、表征了8个半三明治结构环戊二烯基金属铱配合物[(η5-Cpx)Ir(C^N)Cl],其中Cpx分别为四甲基环戊二烯基(C5Me4H),五甲基环戊二烯基(Cp*),四甲基(苯基)环戊二烯基(Cpxph),四甲基(联苯)环戊二烯基(Cpxbiph),C^N为苯亚甲基甲胺(BIMA),N-(4-甲氧基苯亚甲基)苯胺(MBIA)。测定了其中3个配合物的单晶结构。所有配合物对Hela人宫颈癌细胞显示出很强的细胞毒性,IC50值为1.7~32.9μmol·L-1。经检测Cpx铱配合物的抗癌活性顺序为Cpxbiph>Cpxph>C5Me4H>Cp*。配合物[(η5-Cpxbiph)Ir(BIMA)Cl](A4)和[(η5-Cpxbiph)Ir(MBIA)Cl](B4)表现出了最高的抗癌活性,比临床铂类药物顺铂活性高4倍以上。经检测,铱配合物A1~B4不与9-甲基腺嘌呤和9-乙基鸟嘌呤反应,与pBR322 DNA也没有作用,但这些配合物能够作为氢转移催化剂,将辅酶NADH转化为NAD+。机理研究表明配合物A4、B4处理Hela细胞时会引起明显的细胞凋亡和细胞周期的变化,并大幅增加细胞内活性氧(ROS)的水平。
半三明治配合物;环戊二烯基配体;铱;抗癌药物;ROS水平;细胞凋亡;细胞周期;还原态烟酰胺腺嘌呤二核苷酸
Chemotherapy with Pt complexes is one of the main pillars in the treatmentof cancer today[1].Despite their tremendous success,these platinum compounds suffer from twomain disadvantages:they are inefficient againstplatinum-resistant tumors,and they have severe side effects such as nephron toxicity[2-3].The clinical success and drawbacks of Pt anticancer drugs have stimulated the exploration of othermetal-based anticancer compounds[4],in particular other platinum complexes[5]and some group 8metal complexes containing iron[6-8],ruthenium[9-12],rhodium[13-14],osmium[15],which showed promising anticancer activity both in vitro and in vivo.
Half-sandwich cyclopentadienyl(Cpx)Ir complexes have been successfully applied in traditional domains encompassingorganic transformations[16-18],catalysis[19-23]. Very recently,potential biological applications of iridium compounds have attracted much attention[24-27]. A number of Cp*Ircomplexes of the type[(η5-Cp*) Ir(XY)Cl]0/+,where XY=N^N-bound 2,2-bipyridine(bpy) or N^O-bound ligands[28],have been reported to be inactive toward A2780 human ovarian cancer cells. However,the electronic and steric properties of the ligands can have amajor effect on the chemical and biological activities of transition metal complexes.We reported that the potency toward cancer cells increased with additional phenyl substitution on Cp*, and the anticancer activity can be improved significantly by replacement of one atom,changing neutral N^N-chelating ligand(bpy)with negatively charged C^N-chelating ligand 2-phenylpyridine(phpy), which leading to increased cellular uptake and nucleobasebinding[29-30].This finding has led us tomake detailed investigations on CpxIrcomplexes with C^N-bound ligands and to study the influence of phenyl-or biphenyl-substituted Cp*on their chemical and biological properties.
Oxidative stress caused by the generation of reactive oxygen species(ROS)is an effectivemethod of killing cancer cells.ROS are produced in a wide range of physiological processes,in particular by mitochondria,and play valuable roles in cellular signaling.Such stress would have a smaller effect on redox control in normal cells.Organometallic iridiumcomplexes and coppercomplexes have been reported to be effective ROS inducer[31-33].
In the work reported here,eight half-sandwich complexes of the type(η5-Cpx)Ir(C^N)Cl,where Cpxis tetramethylcyclopentadienyl(C5Me4H),Cp*,tetramethyl (phenyl)cyclopentadienyl(Cpxph)or tetramethyl (biphenyl)cyclopentadienyl(Cpxbiph),and the C,N-chelating ligands are N-benzylidenemethylamine(BIMA) and N-(4-methoxybenzylidene)aniline(MBIA)(Chart 1),were designed,synthesized and characterized,and their antiproliferative activity against cancer cells and mechanism of action was investigated.To the best of our knowledge,this appears to be the first report of C5Me4H iridium anticancer agents.The results suggest that this new class of organometallic Ircomplexes iswell suited for development as anticancer agents.
1 Experimental
1.1 M aterials
IrCl3·3H2O,butyllithium solution(1.6 mol·L-1in hexane),1,2,3,4,5-pentamethylcyclopentadiene(95%), 2,3,4,5-tetramethyl-2-cyclopentenone(95%),phenylmagnesium bromide,4-bromobiphenyl,trimethyl(2,3, 4,5-tetramethyl-2,4-cyclopentadien-1-yl)silane(97%), N-benzylidenemethylamine(BIMA),N-(4-methoxybenzylidene)aniline(MBIA),9-ethylguanine and 9-methyladeninewere purchased from Sigma-Aldrich.CpxphH[34], CpxbiphH[28],dimer2[35],A2[36]and B2[37]were prepared according to literature methods.For the biological experiments,DMEM medium,fetal bovine serum, penicillin/streptomycin mixture,trypsin/EDTA,and phosphate-buffered saline(PBS)were purchased from Sangon Biotech.Testing compounds was dissolved in DMSO and diluted with the tissue culture medium before use.
1.2 Instruments and methods
1.2.1 X-ray crystallography
All diffraction data were obtained on a Bruker Smart Apex CCD diffractometer equipped with graphite -monochromated Mo Kαradiation(λ=0.071 073 nm). Absorption corrections were applied using SADABS[38]program.The crystalsweremounted in oil and held at295Kwith theOxford Cryosystem Cobra.Thestructures were solved by directmethodsusing SHELXS(TREF)[39]with additional light atoms found by Fouriermethods. Complexes dimer1,A3 and B3 were refined against F2using SHELXL,and hydrogen atoms were added at calculated positions and refined riding on their parent atoms.Crystallographic data are shown in Table S1, and selected bond lengths and angles are listed in Table S2.
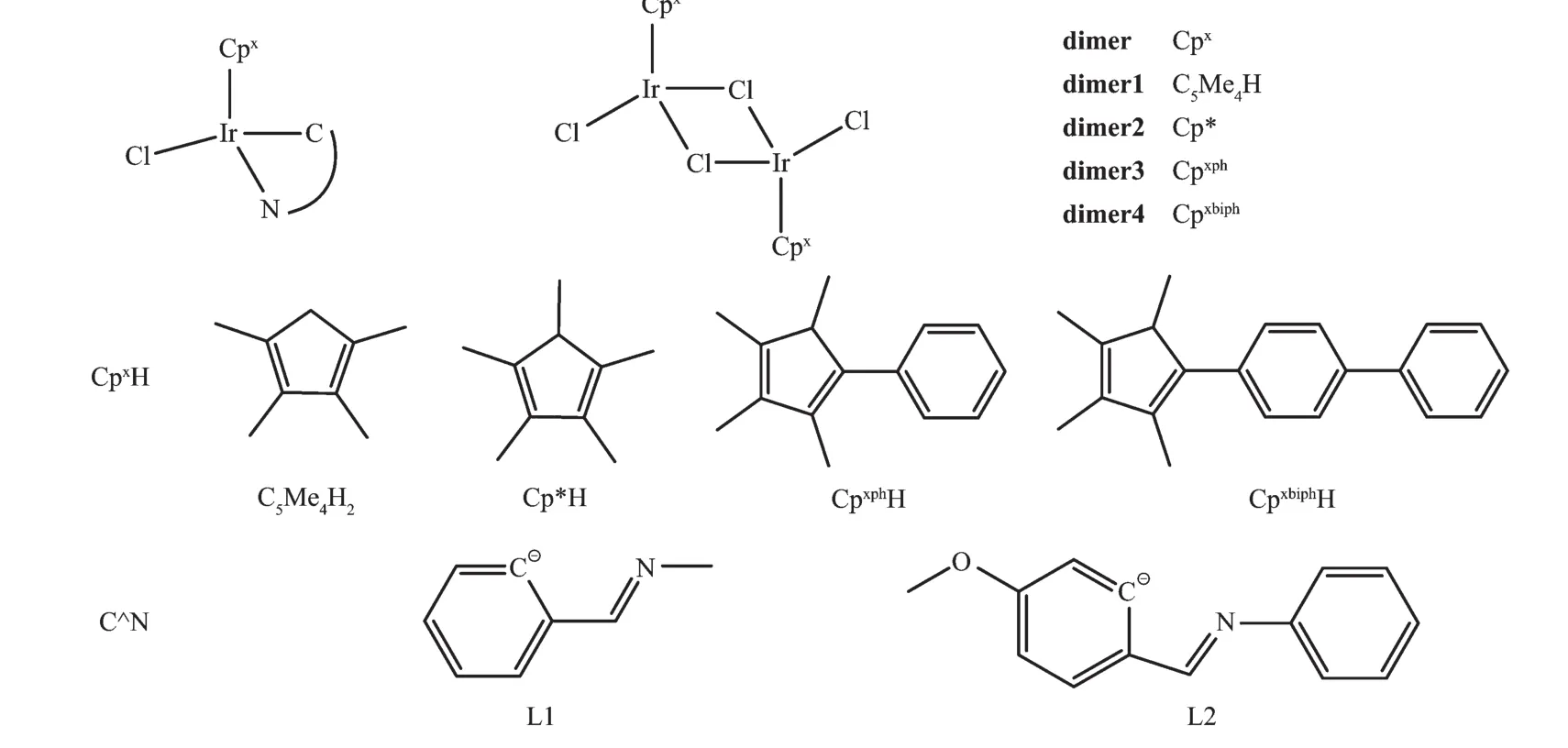
Chart 1 Iridium cyclopentadienyl com plexes studied in thiswork
CCDC:1528262,A3;1528263,B3;1538264, dimer1.
1.2.2 Characterization
1H NMR spectrawereacquired at298K on Bruker -500MHz spectrometers.1H NMR chemicalshiftswere internally referenced to CDCl3(δ7.26)for chloroformd1,CHD2OD(δ3.33)for methanol-d4.Electrospray ionization mass spectra(ESI-MS)were obtained on a Bruker Esquire 2000 ion trap spectrometer.For UVVis spectra,a TU-1901 UV-Vis recording spectrophotometer was used with 1 cm path-length quartz cuvettes(3mL).
1.2.3 Reaction with NADH
The reaction of complexes A1~B4(ca.0.8μmol ·L-1)with NADH(87μmol·L-1,pH=6.7)in 5%(V/V) CH3OH aqueous solution wasmonitored by UV-Vis at 298 K after 8 h.The reaction between complex A2 (0.25mmol·L-1)and NADH(3.5 times the amount of complex)in CHD2OD/D2O(1∶1,V/V)at 298 K was detected by1H NMR.TON was calculated from the difference in NADH concentration after 8 h divided by the concentration of iridium catalyst.The concentration of NADH was obtained using the extinction coefficientε339=6 220 L·mol-1·cm-1.
1.2.4 Interactionswith nucleobases
The reaction of complexes A1~B4(ca.1mmol· L-1)with nucleobases typically involved addition of a solution containing 1 times the amount of nucleobase in D2O to an equilibrium solution of complexes A1~B4 in 40%(V/V)CHD2OD/D2O.1H NMR spectra of these solutions were recorded at 298 K after varioustime intervals.
1.2.5 Cleavage plasmid DNA
Gel electrophoresis experiments were carried out with pBR 322 DNA,in 0.8%agarose solution,at 25 V·cm-1for 1.5 h using TAE buffer(40mol·L-1Tris,1 mmol·L-1EDTA(disodium salt),pH 8.3).The pBR 322 DNA was stained with 0.5 mg·mL-1GelRed.The cleavage reactions were quenched by the addition of bromophenol blue.The cleavage products were irradiated at room temperature with a UV lamp(590 nm).
1.2.6 Cell culture
Hela cervical carcinoma cells were obtained from Shanghai Institute of Biochemistry and Cell Biology (SIBCB)and were grown in Dubelco′s Modified Eagle Medium(DMEM).Allmedia were supplemented with 10%fetal bovine serum,and 1%penicillin-streptomycin solution.All cells were grown at 310 K in a humidified incubatorundera5%(V/V)CO2atmosphere.
1.2.7 In vitro growth inhibition assay(MTT Assay)
After plating 5000 Hela cells per well in 96-well plates,the cells were preincubated in drug-freemedia at 310 K for 24 h before adding different concentrations of the compounds to be tested.In order to prepare the stock solution of the drug,the solid complex was dissolved in DMSO.This stock was further diluted using cell culture medium until working concentrations were achieved.The drug exposure period was 24 h.Subsequently,15μL of 5 mg·mL-1MTT solution was added to form a purple formazan.Afterwards,100μL of dimethyl sulfoxide (DMSO)was transferred into each well to dissolve the purple formazan,and results were measured using a microplate reader(DNM-9606,Perlong Medical, Beijing,China)atan absorbance of 570 nm.Each well was triplicated and each experiment repeated at least three times.IC50values are quoted asmean±SEM.
1.2.8 Cell cycle analysis
Hela cells at 1.5×106per well were seeded in a six-well plate.Cells were preincubated in drug-free media at 310 K for 24 h,after which drugs were added at concentrations of 0.25IC50,0.5IC50,and IC50. After 24 h of drug exposure,supernatants were removed by suction and cells were washed with PBS. Finally,cells were harvested using trypsin-EDTA and fixed for 24 h using cold 70%(V/V)ethanol.DNA staining was achieved by resuspending the cell pellets in PBS containing propidium iodide(PI)and RNAse. Cell pellets were washed and resuspended in PBS before being analyzed in a flow cytometer(ACEA NovoCyte,Hangzhou,China)using excitation of DNA-bound PI at 488 nm,with emission at 585 nm.The cell cycle distribution is shown as the percentage of cells containing G0/G1,S and G2/M DNA as identified by propidium iodide staining.
1.2.9 Induction of apoptosis
Flow cytometry analysis of apoptotic populations of Hela cells caused by exposure to iridium complexes was carried out using the Annexin V-FITC Apoptosis Detection Kit(Beyotime Institute of Biotechnology, China)according to the supplier′s instructions.Briefly, Hela cells(7.5×105per well)were seeded in a sixwell plate.Cellswere preincubated in drug-freemedia at 310 K for 24 h,after which drugs were added at concentrations of IC50and 2IC50.After 24 h of drug exposure,cellswere collected,washed once with PBS, and resuspended in 195μL of Annexin V-FITC binding buffer which was then added to 5μL of Annexin V-FITC and 10μL of PI,and then incubated at room temperature in the dark for 15 min. Subsequently,the buffer placed in an ice bath in the dark.The samples were analyzed by a flow cytometer (ACEA NovoCyte,Hangzhou,China).
1.2.10 ROS determination
Flow cytometry analysis of ROS generation in Hela cells caused by exposure to iridium complexes was carried out using the Reactive Oxygen Species Assay Kit(Beyotime Institute of Biotechnology, Shanghai,China)according to the supplier′s instructions.Briefly,1.5×106Hela cells perwellwere seeded in a six-well plate.Cells were preincubated in drugfree media at 310 K for 24 h in a 5%(V/V)CO2humidified atmosphere,and then drugs were added at concentrations of 0.25IC50and 0.5IC50.After 24 h of drug exposure,cells were washed twice with PBS and then incubated with the DCFH-DA probe(10μmol· L-1)at 37℃for 30 min,and then washed tripleimmediately with PBS.The fluorescence intensity was analyzed by flow cytometry(ACEA NovoCyte, Hangzhou,China).At all times,samples were kept under dark conditions to avoid light-induced ROS production.
1.3 Synthesis
[(η5-Cpxph)IrCl2]2(dimer3).A solution ofCpxphH(1.0 g,5.0 mmol)and IrCl3·3H2O(0.5 g,1.4 mmol)was pretreated with ultrasonic dissolving in an N2atmosphere and reacted with sonication in PTFE inner tank at 120℃for 20min and 150℃for 20min.The reaction mixture was allowed to cool to ambient temperature and pressure and the red-orange precipitate was filtered off.The volume of the dark red filtrate was reduced to ca.15 mL on a rotary evaporator.Upon cooling to ambient temperature,an orange precipitate appeared which was collected by filtration.The productwas washed with methanol and diethyl ether and dried in air.Yield:0.45 g(58.5%).1H NMR(500 MHz,CDCl3)δ7.57(dd,J=6.5,3.0 Hz, 4H),7.41~7.32(m,6H),1.73(s,12H),1.63(s,12H). Anal.Calcd.for C30H34Ir2Cl4(%):C,39.13;H,3.72. Found(%):C,39.14;H,3.68.
[(η5-Cpxbiph)IrCl2]2(dimer4).The synthesis was performed as for dimer3 using CpxbiphH(0.5 g,1.8 mmol)and IrCl3·3H2O(0.2 g,0.7 mmol).Yield:0.13 g(35.8%).1H NMR(500 MHz,CDCl3)δ7.65(d,J= 8.1 Hz,4H),7.62~7.48(m,8H),7.45(dd,J=15.1,7.6 Hz,4H),7.36(t,J=7.3 Hz,2H),1.75(s,12H),1.70(d, J=12.5 Hz,12H).Anal.Calcd.for C42H42Ir2Cl4(%):C, 47.01;H,3.95.Found(%):C,46.87;H,3.75.
[(η5-C5Me4H)IrCl2]2(dimer1).The synthesis was performed as for dimer3 using trimethyl(2,3,4,5-tetramethyl-2,4-cyclopentadien-1-yl)silane(0.7 mL,3 mmol)and IrCl3·3H2O(0.33 g,1.0 mmol).Yield:0.17 g(18.4%).1H NMR(500 MHz,CDCl3)δ5.27(s,2H), 1.68(s,12H),1.63(s,12H).Anal.Calcd.forC18H26Ir2Cl4(%):C,28.13;H,3.41.Found:C,28.25;H,3.56.
[(η5-C5Me4H)Ir(BIMA)Cl](A1).A solution of chloride complex dimer1(46.5 mg,0.06 mmol),N-benzylidenemethylamine(14.3 mg,0.12 mmol),and sodium acetate(49.2mg,0.6mmol),and benzaldehyde (trace)in 20 mL of CH2Cl2was stirred for 5 h at ambient temperature in N2atmosphere.The solution was filtered through celite.The filtrate was evaporated to dryness on a rotary evaporator and washed with diethyl ether and hexane.The product was recrystallized from CH3OH.Yield:19 mg(35.1%).1H NMR (500 MHz,CDCl3)δ8.25(s,1H),7.84(d,J=7.6 Hz, 1H),7.52(d,J=7.5 Hz,1H),7.13(s,1H),6.98(s,1H), 4.78(s,1H),4.01(s,3H),1.78(d,J=6.9 Hz,9H),1.72 (s,3H).MS(MeOH):m/z=508.1([(η5-C5Me4H)Ir(BIMA) Cl]+K+).Anal.Calcd.for C17H21IrNCl(%):C,43.72;H, 4.53;N,3.00.Found(%):C,43.78;H,4.44;N,2.88.
[(η5-Cpxph)Ir(BIMA)Cl](A3).The synthesis was performed as for A1 using complex dimer3(46 mg, 0.05 mmol),N-benzylidenemethylamine(12 mg,0.1 mmol),and sodium acetate(41 mg,0.5 mmol),and benzaldehyde(trace).Yield:38 mg(70.0%).1H NMR (500 MHz,CDCl3)δ8.26(d,J=1.3 Hz,1H),7.62(d, J=7.5Hz,1H),7.53(dd,J=7.5,1.2Hz,1H),7.39~7.28 (m,5H),7.08(tt,J=13.0,6.5 Hz,1H),6.98(td,J=7.4, 1.0Hz,1H),3.73(d,J=1.3 Hz,3H),1.87(s,3H),1.80 (d,J=1.4Hz,6H),1.67(s,3H).MS(MeOH):m/z=583.8 ([(η5-Cpxph)Ir(BIMA)Cl]+K+).Anal.Calcd.for C23H25Ir NCl(%):C,50.86;H,4.64;N,2.58.Found(%):C,50.82; H,4.69;N,2.53.
[(η5-Cpxbiph)Ir(BIMA)Cl](A4).The synthesis was performed as for A1 using complex dimer4(53 mg, 0.05 mmol),N-benzylidenemethylamine(12 mg,0.1 mmol),and sodium acetate(41 mg,0.5 mmol),and benzaldehyde(trace).Yield:24 mg(38.8%).1H NMR (500 MHz,CDCl3)δ8.28(d,J=1.2 Hz,1H),7.64(dd, J=9.1,7.7 Hz,3H),7.58(d,J=8.3 Hz,2H),7.54(d, J=7.5 Hz,1H),7.51~7.40(m,4H),7.36(t,J=7.4 Hz, 1H),7.10(td,J=7.5,1.3 Hz,1H),6.99(dd,J=7.4,6.5 Hz,1H),3.77(t,J=7.8 Hz,3H),1.88(s,J=10.2 Hz, 3H),1.84(s,3H),1.81(s,3H),1.72(s,3H).MS(MeOH): m/z=583.8([C23H25IrNCl]+K+).Anal.Calcd.for C29H29Ir NCl(%):C,56.25;H,4.72;N,2.26.Found(%):C, 56.29;H,4.77;N,2.20.
[(η5-C5Me4H)Ir(MBIA)Cl](B1).The synthesis was performed as for A1 using complex dimer1(46.5 mg, 0.06mmol),N-(4-methoxybenzylidene)aniline(25.5mg, 0.12 mmol),and sodium acetate(49.2 mg,0.6 mmol), and benzaldehyde(trace).Yield:27mg(48.3%).1HNMR(500 MHz,CDCl3)δ8.23(s,1H),7.66~7.57(m, 3H),7.43(d,J=2.4 Hz,1H),7.37(t,J=7.8 Hz,3H), 7.28(d,J=7.4 Hz,1H),6.61(dd,J=8.4,2.4 Hz,1H), 3.91(s,3H),1.62(s,3H),1.56(s,3H),1.53(s,3H), 1.44(s,3H).MS(MeOH):m/z=598.8([(η5-C5Me4H)Ir (MBIA)Cl]+K+).Anal.Calcd.for C23H25IrNClO(%):C, 49.41;H,4.51;N,2.51.Found(%):C,49.47;H,4.46; N,2.56.
[(η5-Cpxph)Ir(MBIA)Cl](B3).The synthesis was performed as for A1 using complex dimer3(46 mg, 0.05 mmol),N-(4-methoxybenzylidene)aniline(22 mg, 0.1mmol),and sodium acetate(41mg,0.5mmol),and benzaldehyde(trace).Yield:47 mg(74.0%).1H NMR (500 MHz,CDCl3)δ8.22(s,1H),7.58(d,J=8.3 Hz, 1H),7.50(d,J=7.3 Hz,2H),7.39~7.28(m,7H),7.23 (d,J=7.4 Hz,1H),7.06~7.02(m,1H),6.57(dd,J=8.4, 2.4Hz,1H),3.61(s,3H),1.70(s,3H),1.48(s,6H),1.35 (s,3H).MS(MeOH):m/z=675.7([(η5-Cpxph)Ir(MBIA) Cl]+K+).Anal.Calcd.for C29H29IrNClO(%):C,54.83; H,4.60;N,2.21.Found(%):C,54.75;H,4.50;N,2.26.
[(η5-Cpxbiph)Ir(MBIA)Cl](B4).The synthesis was performed as for A1 using complex dimer4(53 mg, 0.05 mmol),N-(4-methoxybenzylidene)aniline(22 mg, 0.1 mmol),and sodium acetate(41 mg,0.5 mmol), and benzaldehyde(trace).Yield:23mg(32.3%).1H NMR(500 MHz,CDCl3)δ8.22(s,1H),7.64~7.54(m, 5H),7.52(dd,J=8.3,1.1 Hz,2H),7.49~7.39(m,4H), 7.39~7.31(m,3H),7.26~7.20(m,1H),7.05(dd,J= 11.3,7.1Hz,1H),6.57(dd,J=8.4,2.4Hz,1H),3.61(d, J=5.3 Hz,3H),1.76(d,J=6.8 Hz,3H),1.49(s,6H), 1.41(s,3H).MS(MeOH):m/z=675.7([C29H29IrNOCl]+ K+).Anal.Calcd.for C35H33IrNClO(%):C,59.10;H, 4.68;N,1.97.Found(%):C,59.18;H,4.64;N,1.92.
2 Results and discussion
2.1 Synthesis
dimer1,dimer2,dimer3 and dimer4 were synthesized by using a Tank Eco Initiator microwave synthesizer.Compared to the heating reflux method, the yield was improved effectively[28].Eight Irhalfsandwich complexes(Chart 1)of the type[(η5-Cpx)Ir (C^N)Cl],where Cpxis C5Me4H,Cp*or its phenyl(Cpxph) or biphenyl(Cpxbiph)derivatives,and the C^N-chelating ligands are N-benzylidenemethylamine(BIMA)and N-(4-methoxybenzylidene)aniline(MBIA),were synthesized by reaction of the C^N-chelating ligands with the iridium precursors dimer1~dimer4 in dichloromethane in the presence of sodium acetate as a base. All of the synthesized complexes were fully characterized by1H NMR spectroscopy,mass spectrometry and elemental analysis.Introduction of phenyl substituents on the Cp*ring decreased the reaction yields significantly.At the beginning,an iridium silane dimer[(η5-C5Me4SiMe3)IrCl2]2was tried to synthesized by reaction of trimethyl(2,3,4,5-tetramethyl-2,4-cyclopentadien-1-yl)silane with IrCl3·3H2O by microwave heating.Unfortunately,thismethod led to loss of silanewith formation of dimer1.
2.2 X-ray crystal structures
The X-ray crystal structures of dimer1,A3 and B3 were determined.The structures and atomnumbering schemes are shown in Fig.1.Complexes A3 and B3 adopt the expected half-sandwich pseudooctahedral“three-legged piano-stool”geometry with the iridium bound to aη5-cyclopentadienyl ligand(Ir to ring centroid distances of 0.175 0,0.182 2 and 0.183 1 nm,respectively).The Ir-Clbond distancesare 0.238 36(19),0.239 21(10)and 0.240 30(9)nm for dimer1,A3 and B3,respectively.The Ir-C(chelating ligand)bond lengths in A3 and B3(0.203 7(4)and 0.203 3(4)nm,respectively)are significantly shorter than the Ir-N bond lengths(0.208 4(3)and 0.209 5(3) nm,respectively).
Thebond distancesand angles in dimer1 compare well to those found for the corresponding Cp* analogue dimer2[40].The Ir-Cl(bridging)-Ir angle in dimer1 is 1.11°more acute and the Cl(bridging)-Ir-Cl (bridging)angle in dimer1 is 0.81°more obtuse, respectively,than those of the Cp*analogue.The Ir-Cl bound lengths in complex A3(0.239 21(10)nm)is slightly shorter than complex B3(0.240 30(9)nm) (Table S2).The Ir-Cl bond length in A3(0.239 21(10) nm)is similar to that in A2(0.239 50(6)nm)[41].The Ir-Cl bound length in complexes B3(0.240 30(9)nm) is similar to that in complex B2(0.240 38(5)nm)[37]. These results suggest that the introduction of phenylsubstituent on the Cp*ring does not give rise to significant change in structure.
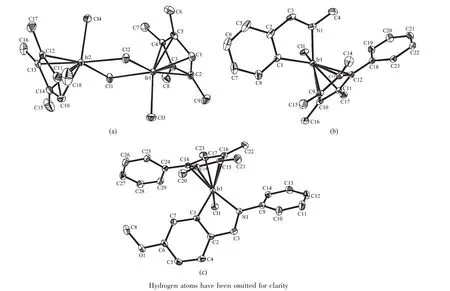
Fig.1 X-ray crystal structures and atom-numbering schemes for complexes dimer1,A3 and B3 with thermal ellipsoids drawn at50%probability
2.3 Interaction w ith nucleobases
Because DNA is a potential target site for transition metal anticancer complexes,the binding of 9-ethylguanine(9-EtG)and 9-mehyladenine(9-MeA) to complexes A1~B4 was studied.The addition of 1 times the amountof 9-MeA or 9-EtG to an equilibrium solution of A1~B4(1.0mmol·L-1)in 20%(V/V)MeOH-d4/D2O at 298 K resulted in no additional1H NMR peaks over a period of 24 h(Fig.S1),indicating no reaction with 9-MeA or 9-EtG was observed for A1~B4(Table S3).
2.4 Cleavage plasm id DNA
The DNA binding ability of complexes A1~B4 was studied through agarose gel electrophoresis[42]of supercoiled pBR 322 plasmid DNA[43]in Tris buffer.It is known that the binding of unwinding agents to the closed circular DNA can reduce its super helical density and hence decrease its rate of migration in agarose gel.However,for complexes A1~B4,no DNA cleavage was observed even at concentration of 100 μmol·L-1(Fig.S2).Thus DNA is not likely to be a target for this type of complexes[31].
2.5 Antiproliferative activity
The activity of complexes A1~B4 toward Hela human cervical cancer cells was investigated(Table 1).All of the eight complexes A1~B4 were active, displaying IC50values of 1.7~32.9μmol·L-1.C5Me4Hcomplexes A1,B1 and Cp*complexes A2,B2 showed good activity,displaying IC50values of 13.0~32.9μmol ·L-1.Complex A3 containing Cpxphshowed similar activity compare tocisplatin(IC50=7.5μmol·L-1).Cpxbiphcomplexes A4,B4 and B3 exhibited potent anticancer activity with IC50values of 1.7~3.5μmol·L-1,respectively,ca.two to four timesmore active than cisplatin against the Hela cell line.For all complexes,the trend of increasing of activity with increasing phenyl substitution was the same:Cpxbiph>Cpxph>C5Me4H>Cp*. The introduction of phenyl or biphenyl substituents onto the tetramethylcyclopentadienyl ring results in a significant increase in cytotoxicity compared to the parent Cp*complex.This result is consistentwith our previously report[28,44].Interestingly,however,loss of one methyl group on the Cp*almost doubled the antiproliferative activity.To the bestof our knowledge, this appears to be the first report of C5Me4H iridium anticancer agents.

Table 1 Inhibition of grow th of Hela human cervical cancer cells by complexes A1~B4 and cisplatin
2.6 Reaction w ith NADH
Coenzyme NADH and NAD+plays a key role in numerous biocatalyzed processes.Previously,we have shown that NADH can donate a hydride to aqua Ircyclopentadienyl complexes and can produce ROS H2O2thus provide a pathway to an oxidantmechanism of action[33].We have investigated whether complexes A1~B4 react with NADH and thus provide a redox pathway.When NADH(3.5 times the amount of complex)was added to a 0.25 mmol·L-1solution of complex A2,a sharp singlet atδ-16.67 was observed in the1H NMR spectrum within ten minutes;this resonance corresponds to the Irhydrido complex A2 (Fig.2a).Simultaneously,NADH was converted into its oxidized form NAD+.These data suggest that complexA2 can accept a hydride from NADH.Similar results were obtained for the reaction of NADH with other complexes(Fig.S2).Strikingly,data from UV-Vis spectroscopy suggested that all the complexes can act as catalysts for hydride transfer from NADH(Fig.2b and Fig.S3).The turnover numbers(TON)in thiswork is defined as the amounts of NADH that a mole of catalyst can convert within 8 h.TONs of different complexes can be calculated by measuring the absorption difference at 339 nm(Fig.2c,Table S4). Themaximum TON reached 31 mol·molcat-1after 8 h for complex A2.
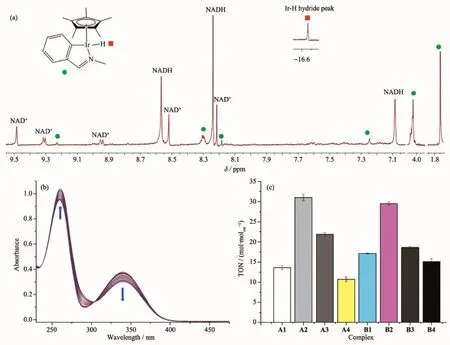
Fig.2 (a)1H NMR spectra for the reaction between complex A2(0.25mm)and NADH(3.5 times the amountof complex)in CD3OD/D2O(1∶1,V/V)at298 K;(b)UV-Vis spectra for the reaction of NADH(87μmol·L-1)with complex A2 (0.8μmol·L-1)in MeOH/H2O(1.6∶98.4,V/V)at298 K for 8 h;(c)TONs of complexes A1~B4
Previously,we have shown that organometallic iridiumcomplexes are particularly effective oxidant catalysts which have anticancer activity,offering a new strategy for the design ofmetal-based anticancer drugs[33,45-46].Other metal based compounds have also been reported to be effective biological catalysis[47]. For example,manganesemacrocyclescan act as superoxide dismutase mimics and decompose superoxide into O2and H2O2in cells[48].Iron porphyrin complexes can catalyse the reduction of azides toimines[49],and copper peptide complexes can catalyse the degradation of RNA in hepatitismodels[50].Cobalt complexes,which cleave peptide bonds,can decompose amyloids present in diseases such as Alzheimer′s or Parkinson′s[51].In particular,organometallic ruthenium compounds have shown success in the last few years.For example, Noyori-type catalytic transfer hydrogenation can be achieved in living cells,using Ruarene complexes with a chelated sulfonylethylamine ligand and formate as the hydride donor to convert coenzyme NAD+into NADH,and thereby modulate the NAD+/NADH redox couple[52].
2.7 Cell cycle study
We performed cell cycle arrest analysis for complexes A4 and B4 toward Hela cells by flow cytometry to determine whether the induced cell growth inhibition was the result of cell cycle arrest.In comparison to the control population,the cell cycle progression was analyzed at concentrations of 0.25IC50, 0.5IC50and IC50of complexes A4 and B4 for 24 h. There was an increase in the G1proportion of Hela cell lines(from 55.0%to 68.0%and from 55.0%to 65.0%,respectively)(Fig.3,Table S5),indicating that the cells cycleswere blocked in the G1phase.
2.8 Apoptosis assay
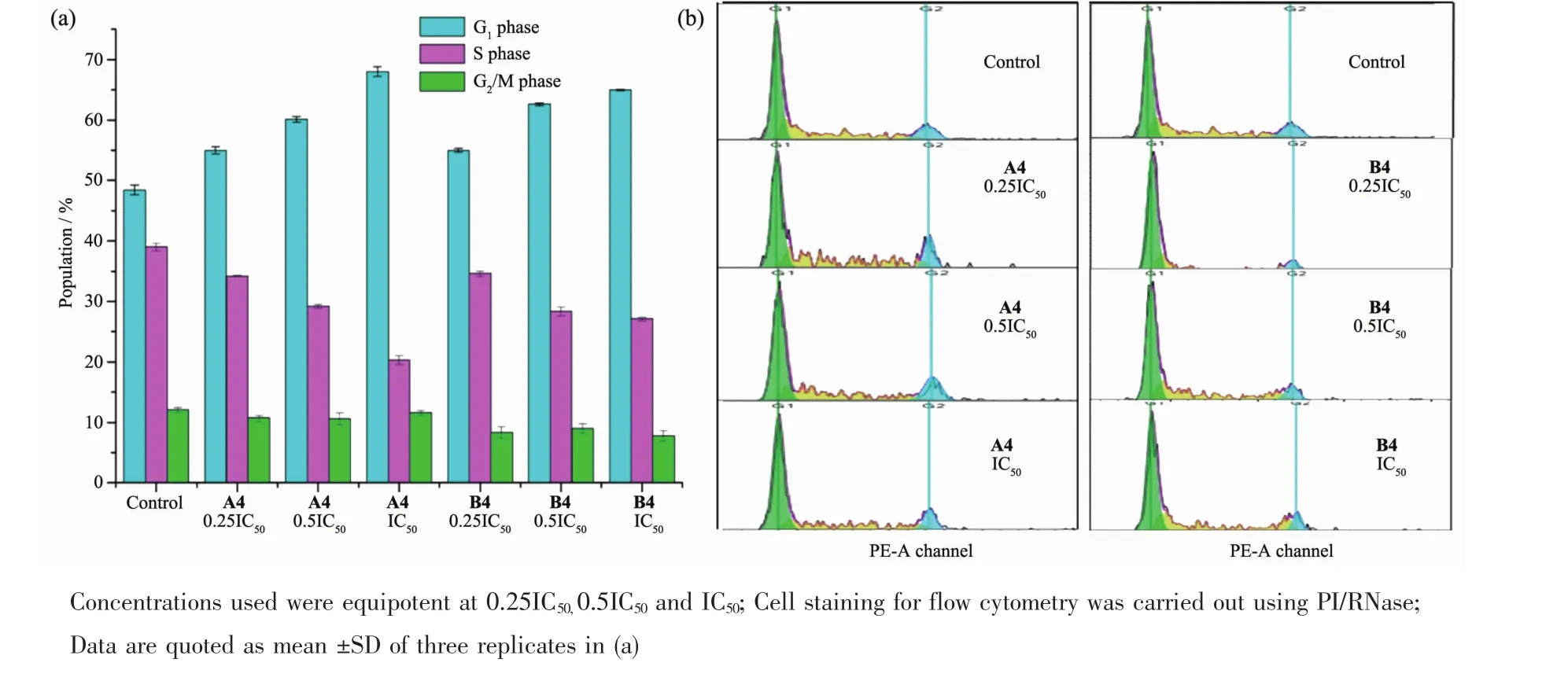
Fig.3 Cell cycle analysis of Hela cells after 24 h of exposure to complexes A4 and B4 at310 K;(a)Cell populations in each cell cycle phase for control(cells untreated)and complexes A4 and B4;(b)FL2 histogram for control(cells untreated) and complexes A4 and B4
In order to investigate whether the reduction in cell viability observed by the MTT assay is based on apoptosis,Hela cells were treated with complexes A4 and B4 at equipotent concentrations of IC50and 2IC50for 24 h,then stained with Annexin V/propidiumiodide and analyzed by flow cytometry[53].This allowed determination of cell populations as viable(unstained, only self-fluorescence),early apoptosis(stained by Annexin V only,green fluorescence),late apoptosis (stained by Annexin V and PI,green and red fluorescence),and nonviable(stained by PIonly,red fluorescence).Upon exposure of the cells to complex A4 at a concentration of IC50after 24 h,only around 0.77%of Hela cells remained in early apoptotic phase and 4.95%of cells were in the late apoptotic phase (Fig.4 and Table S6).However,at 2IC50,a total of 26.16%(early apoptotic+late apoptotic)cells were undergoing apoptosis,whereas untreated cells remained 93.90%viable,indicating obvious inductionof apoptosis at2IC50.Similarly,apoptosiswas observed when Hela cells were exposed to the complex B4 at 2IC50concentration,leading to a total of 27.51%(early apoptotic+late apoptotic)cells undergoing apoptosis. This suggested that cell death was induced by A4 and B4mainly through apoptosis
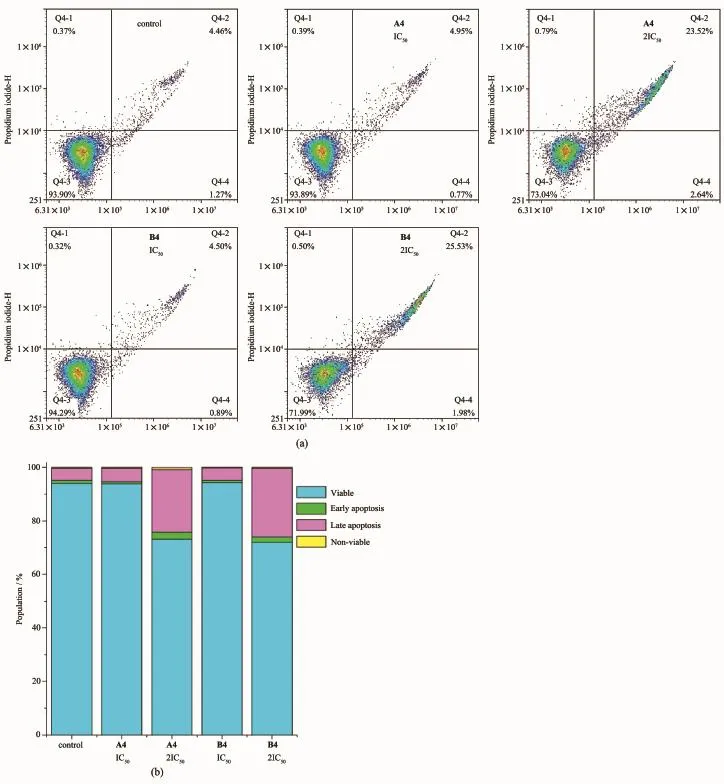
Fig.4 Apoptosis analysis of Hela cells after 24 h of exposure to complexes A4 and B4 at310 K determined by flow cytometry using Annexin V-FITC vs PIstaining at IC50and 2IC50:(a)HeLa cellswere control and treated with different concentrations of Ircomplexes A4 and B4 for 24 h;(B)Populations for cells treated by A4 and B4
2.9 Induction of ROS in Hela cancer cells
Oxidative stress caused by the generation of reactive oxygen species(ROS)is an effectivemethod of killing cancer cells[33].We suggested previously that theantiproliferativemechanism for the iridium pyridine complex is related to ROS generation.Here we determined the level of reactive oxygen species(ROS) in Hela cells induced by complexes A4 and B4 at concentrationsof0.25IC50and 0.5IC50by flow cytometry fluorescence analysis(Fig.5,Table S7).
After 24 h of drug exposure,dramatic increases in ROS levels in cells treated with complexes A4 and B4 was observed.The majority of Hela cancer cells, more than 94%,were in a high ROS levels after exposure to both Ir complexes A4 and B4 even at concentration of 0.25IC50.The concentration dependence of ROS induction was not obvious.These increases in ROS levels may provide a basis for killing cancer cells.
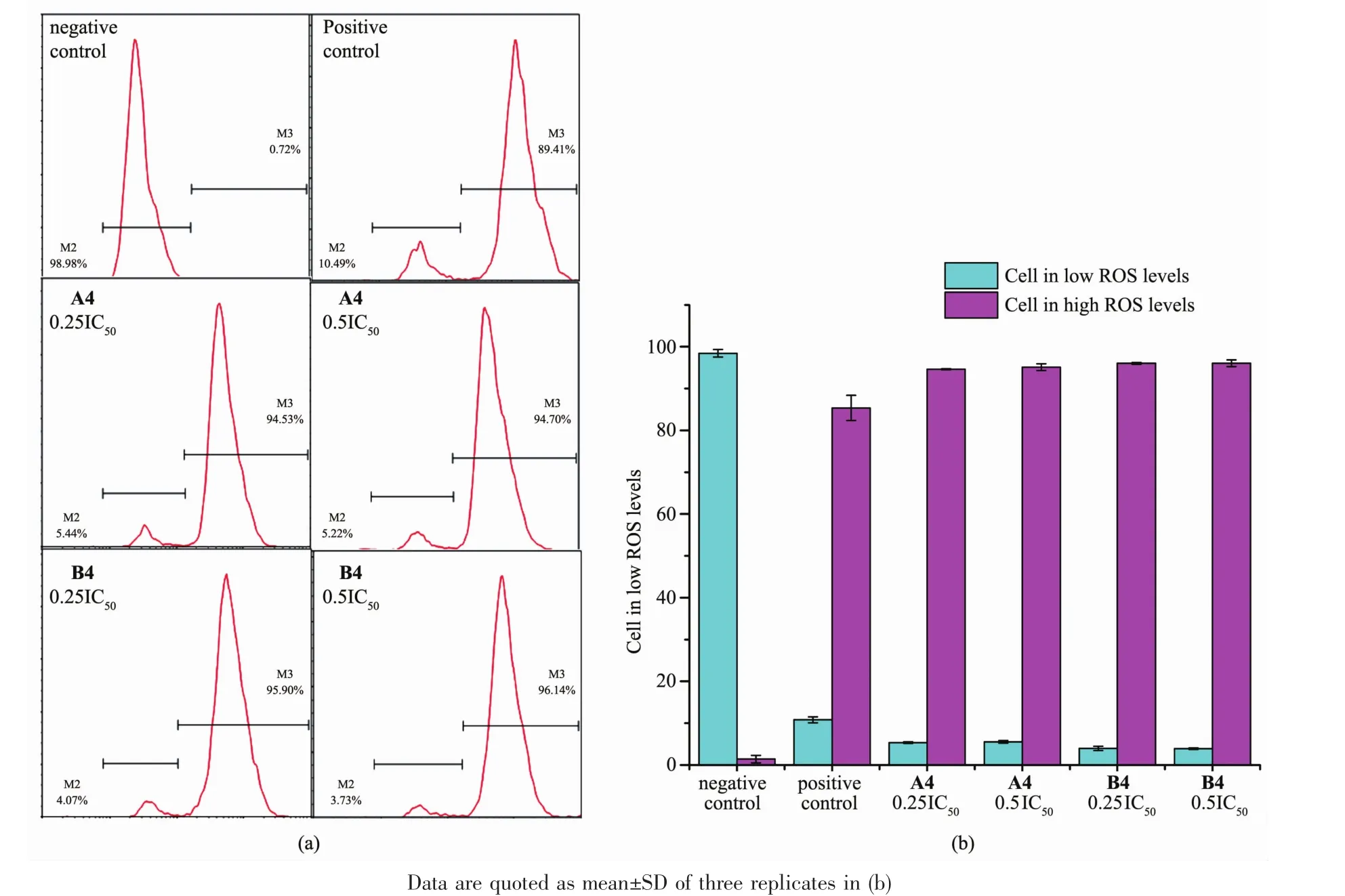
Fig.5 ROS induction in Hela cells treated with complexes A4 and B4 atequipotent concentrations of 0.25IC50and 0.5IC50: (a)FL2 histogram for negative control(cells untreated),positive control and complexes A4 and B4;(b)Populations for cells treated by A4 and B4
3 Conclusions
In thiswork,we have prepared eight new organometallic Ircyclopentadienyl complexes[(η5-Cpx)Ir (C^N)Cl]to explore the effect of cyclopentadienyl ligand and C^N-chelating ligand on their chemical and anticancer activity.The X-ray crystal structures of dimer1,A3 and B3 were determined.This appears to be the first reportofC5Me4H iridium anticanceragents.
All complexes A1~B4 studied here display high potency toward the human Hela cancer cell, comparable to,and for some complexes even higherthan the clinical anticancer drug cisplatin.The anticancer activity can be tuned by varying the cyclopentadienyl ligand,and increased in the order of Cpxbiph>Cpxph>C5Me4H>Cp*.The most active complexes A4 and B4 containing biphenyl substituenton cyclopentadienyl ring,is over 4 timesmore potent than cisplatin against Hela cells.
No distinct nucleobase binding for this type of complexes to 9-MeA and 9-EtG,and no unwinding to the pBR 322 DNA,suggesting that DNA could not be the main target for these complexes.However,all complexes are effective catalysis in transfer hydrogenation converting coenzyme NADH into NAD+. Organometallic iridium complexes are unique in their ability to achieve such redox modulation in living cells[32].In contrast to the DNA-targeting platinum drugs,these organometallic iridium complexes seem to offer the possibility of alternative redoxmechanism of action.Complexes A4 and B4 induce a dramatic increase in the level of ROS in Hela cancer cells after 24 h.Additionally,cell cycle arrest at G1phase and distinct apoptosis were induced when Hela cancer cells were treated with different IC50concentrations of complexes A4 and B4.Our work suggests that this type of iridium complex could be a promising candidate for further evaluation as chemotherapeutic agents for human cancers.
Supporting information isavailable athttp://www.wjhxxb.cn
[1]Jakupec M A,Galanski M,Arion V B,et al.Dalton Trans., 2008:183-194
[2]Dyson P Jand Sava G.Dalton Trans.,2006:1929-1933
[3]Wang X,Wang X,Guo Z.Acc.Chem.Res.,2015,48:2622-2631
[4]Gasser G,Ott I,Metzler-Nolte N.J.Med.Chem.,2011,54(1): 3-25
[5]Wilbuer A,Vlecken D H,Schmitz D J,et al.Angew.Chem. Int.Ed.,2010,49(22):3839-3842
[6]Top S,Vessières A,Leclercq G,et al.Chem.Eur.J.,2003,9 (21):5223-5236
[7]Hamels D,Dansette PM,Hillard E A,et al.Angew.Chem. Int.Ed.,2009,121(48):9124-9126
[8]Tan Y L K,Pigeon P,Hillard E A,et al.Dalton Trans., 2009:10871-10881
[9]Hartinger C G,Zorbas-Seifried S,Jakupec M A,et al.J. Inorg.Biochem.,2006,100(5/6):891-904
[10]Pierroz V,Joshi T,Leonidova A,et al.J.Am.Chem.Soc., 2012,134(50):20376-20387
[11]CHEN Xiang(陈相),CHAO Hui(巢晖),JI Ling-Nian(计亮年).Chinese J.Inorg.Chem.(无机化学学报),2015,31(9): 1667-1677
[12]LIHong(李红),CHAO Hui(巢晖),JING Xiong(蒋雄),et al. Acta Phys.-Chim.Sin.(物理化学学报),2001,17(8):728-732
[13]Dorcier A,Ang W H,Bolao S,et al.Organometallics,2006, 25(17):4090-4096
[14]Markham J,Liang J,Levina A,et al.Eur.J.Inorg.Chem., 2017:1812-1823
[15]Zhou W,Wang X,Hu M,et al.Chem.Sci.,2014,5(7):2761-2770
[16]Davies D L,Al-Duaij O,Fawcett J,et al.Organometallics, 2010,29(6):1413-1420
[17]Schwab P,Grubbs RH,Ziller JW.J.Am.Chem.Soc.,1996, 118(1):100-110
[18]CHEN Yu(陈禹),DU Ke-Jie(杜可杰),CHAO Hui(巢晖), et al.Prog.Chem.(化学进展),2009,21(5):836-844
[19]GUO Li-Hua(郭丽华),CHEN Chang-Le(陈昶乐).Sci. China:Chem.(中国科学:化学),2015,58(11):1663-1673
[20]Guo L,Dai S,Chen C.Polymers,2016,8(2):37(10 pages)
[21]Xiong S Y,Guo L H,Zhang S M,et al.Chin.J.Chem., 2017,DOI:10.1002/cjoc.201600898
[22]Liu Z,Lebrun V,Kitanosono T,et al.Angew.Chem.Int. Ed.,2016:11587-11590
[23]Guo L,Jing X,Xiong S,et al.Polymers,2016,8(11):389(12 pages)
[24]Li SP-Y,Yip A M-H,Liu H-W,et al.Biomaterials,2016, 103:305-313
[25]Lo K K-W.Acc.Chem.Res.,2015,48(12):2985-2995
[26]Zhao Q,Zhang C,Liu S,et al.Sci.Rep.,2015,5:16420(11 pages)
[27]Dörr M,Meggers E.Curr.Opin.Chem.Biol.,2014,19:76-81
[28]Liu Z,Habtemariam A,Pizarro A M,et al.J.Med.Chem., 2011,54(8):3011-3026
[29]Liu Z,Habtemariam A,Pizarro A M,et al.Organometallics, 2011,30(17):4702-4710
[30]Liu Z,Romero-Canelón I,Habtemariam A,et al.Organometallics,2014,33(19):5324-5333
[31]Wang C,Liu J,Tian Z,et al.Dalton Trans.,2017,46:6870-6883
[32]Galluzzi L,Maiuri M C,Vitale I,et al.Cell Death Differ.,2007,14(7):1237-1243
[33]Liu Z,Romero-Canelón I,Qamar B,etal.Angew.Chem.Int. Ed.,2014,53(15):3941-3946
[34]Bjrgvinsson M,Halldorsson S,Arnason I,et al.J.Org. Chem.,1997,544(2):207-215
[35]Tnnemann J,Risse J,Grote Z,et al.Eur.J.Inorg.Chem., 2013:4558-4562
[36]Davies D L,Al-Duaij O,Fawcett J,et al.Dalton Trans., 2003:4132-4138
[37]Li L,Brennessel W W,Jones W D.Organometallics,2009, 28(12):3492-3500
[38]Sheldrick G M.SADABS,University of Göttingen,Germany, 1994.
[39]Sheldrick G M.Acta Crystallogr.Sect.A,1990,A46:467-473
[40]Churchill M R,Julis S A.Inorg.Chem.,1977,16(6):1488-1494
[41]Li L,Brennessel W W,Jones W D.J.Am.Chem.Soc., 2008,130(37):12414-12419
[42]Wang J,Wang X,Song Y,et al.Chem.Sci.,2013,4(6):2605 -2612
[43]Xi P X,Xu Z H,Chen F J,et al.J.Inorg.Biochem.,2009, 103(2):210-218
[44]Liu Z,Sadler P J.Acc.Chem.Res.,2014,47:1174-1185
[45]Liu Z,Deeth R J,Butler JS,et al.Angew.Chem.Int.Ed., 2013,52:4194-4197
[46]Betanzos-Lara S,Liu Z,Habtemariam A,etal.Angew.Chem. Int.Ed.,2012,51:3897-3900
[47]Sasmal P K,Streu CN,Meggers E.Chem.Commun.,2013, 49(16):1581-1587
[48]Filipovi M R,Koh A CW,Arbault S,et al.Angew.Chem. Int.Ed.,2010,49(25):4228-4232
[49]Sasmal P K,Carregal-Romero S,Han A A,et al. ChemBioChem,2012,13(8):1116-1120
[50]Bradford S,Cowan JA.Chem.Commun.,2012,48(25):3118-3120
[51]Lee TY,Suh J.Chem.Soc.Rev.,2009,38(7):1949-1957
[52]Soldevila-Barreda J J,Romero-Canelón I,Habtemariam A, et al.Nat.Commun.,2015,6:6582
[53]WANG Jie(王洁),WANG Zeng-Li(王曾礼),ZHU Yuan-Jue (朱元珏),et al.Chin.J.Intern.Med.(中华内科杂志),1998 (8):28-31
Potent Cyclopentadienyl Iridium Anticancer Comp lexes Containing C^N-chelating Ligands
LU Xiao-Min TIAN Meng TIAN Zhen-Zhen TIAN Lai-Jin LIMeng-Qi HUANG Jing LIU Zhe*
(Institute of Anticancer Agents Development and Theranostic Application,Shandong Provincial Key Laboratory of Life-Organic Analysis,Shandong Provincial Key Laboratory of Pharmaceutical Intermediates and Analysis of NaturalMedicine,Department of Chemistry and Chemical Engineering,Qufu Normal University,Qufu,Shandong 273165,China)
Half-sandwich cyclopentadienyl Ircomplexes of the type[(η5-Cpx)Ir(C^N)Cl],(Cpx=C5Me4H,Cp*, tetramethyl(phenyl)cyclopentadienyl(Cpxph),tetramethyl(biphenyl)cyclopentadienyl(Cpxbiph),C^N=N-benzylidenemethylamine(BIMA),N-(4-methoxybenzylidene)aniline(MBIA)),have been synthesized and characterized.X-ray crystal structures of three complexes have been determined.All complexes showed potent cytotoxicity,with IC50values ranging from 32.9 to 1.7μmol·L-1toward Hela human cervical cancer cells.Their potency is in the trend: Cpxbiph>Cpxph>C5Me4H>Cp*.Complexes[(η5-Cpxbiph)Ir(BIMA)Cl](A4)and[(η5-Cpxbiph)Ir(MBIA)Cl](B4)displayed the highest potency,more than 4 times more active than the clinical platinum drug cisplatin.No binding to nucleobases 9-ethylguanine(9-EtG),9-methyladenine(9-MeA)and pBR 322 DNA were detected.The ability of these iridium complexes to catalytic hydride transfer from the coenzyme NADH to NAD+is studied.Complexes A4 and B4 cause cell apoptosis and arrest cell cycles at G1phase when Hela cancer cells were treated with different IC50concentrations of complexes,and increase the reactive oxygen species(ROS)dramatically,which appears to contribute to the anticancer activity.CCDC:1528262,A3;1528263,B3;1538264,dimer1.
half-sandwich complexes;cyclopentadienyl ligand;iridium;anticancer drug;ROS level;apoptosis;cellcyclearrest;NADH
O614.82+5
A
1001-4861(2017)07-1119-13
10.11862/CJIC.2017.155
2017-04-29。收修改稿日期:2017-05-19。
国家自然科学基金(No.21671118)和泰山学者工程资助项目。*
。E-mail:liuzheqd@163.com

